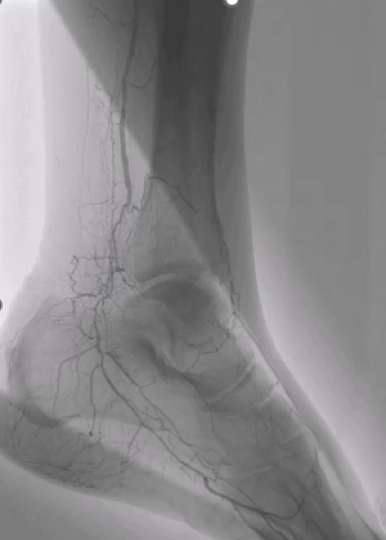
Shockwave L6 Real-World Case Discussion
In a panel discussion at VAM 2023, Drs. Karan Garg, Misty Humphries and Mathew Wooster review their treatment algorithm for tackling calcium in large vessels, the benefits of the larger diameter sizes of Shockwave L6, appropriate IVL sizing for optimal results and real-world Shockwave L6 cases demonstrating its safe and effective use.
In Part 1, Drs. Garg, Humphries and Wooster discuss their large vessel treatment algorithms and the use of the larger Shockwave L6 sizes, while also reviewing an infrarenal EVAR case from Dr. Wooster where Shockwave L6 was used to treat a calcified CFA on the way out.
In Part 2, the physicians expand their conversation to the entire Shockwave IVL peripheral portfolio and review a case from Dr. Humphries where Shockwave L6 was used to effectively modify a calcific iliac artery in order to place a sheath for a successful physician-modified endograft delivery.
The physicians featured are paid consultants for Shockwave Medical.
Peripheral IVL
Shockwave M5+, Shockwave M5, Shockwave S4, Shockwave L6 and Shockwave E8 Safety Information
In the United States: Rx only.
Indications for Use—The Shockwave Medical Intravascular Lithotripsy (IVL) System is intended for lithotripsy-enhanced balloon dilatation of lesions, including calcified lesions, in the peripheral vasculature, including the iliac, femoral, ilio-femoral, popliteal, infra-popliteal, and renal arteries. Not for use in the coronary, carotid or cerebral vasculature.
Contraindications—Do not use if unable to pass 0.014″ (M5, M5+, S4, E8) or 0.018″ (L6) guidewire across the lesion-Not intended for treatment of in-stent restenosis or in coronary, carotid, or cerebrovascular arteries.
Warnings—Only to be used by physicians who are familiar with interventional vascular procedures—Physicians must be trained prior to use of the device—Use the generator in accordance with recommended settings as stated in the Operator’s Manual.
Precautions—use only the recommended balloon inflation medium—Appropriate anticoagulant therapy should be administered by the physician—Decision regarding use of distal protection should be made based on physician assessment of treatment lesion morphology.
Adverse effects–Possible adverse effects consistent with standard angioplasty include–Access site complications–Allergy to contrast or blood thinner–Arterial bypass surgery—Bleeding complications—Death—Fracture of guidewire or device—Hypertension/Hypotension—Infection/sepsis—Placement of a stent—renal failure—Shock/pulmonary edema—target vessel stenosis or occlusion—Vascular complications. Risks unique to the device and its use—Allergy to catheter material(s)— Device malfunction or failure—Excess heat at target site.
Prior to use, please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions and adverse events. www.shockwavemedical.com/ifu