Shockwave Medical Reports Fourth Quarter and Full Year 2019 Financial Results and Provides Full Year 2020 Financial Outlook
SANTA CLARA, Calif., Feb. 13, 2020 (GLOBE NEWSWIRE) — Shockwave Medical, Inc. (Nasdaq: SWAV), a pioneer in the development and commercialization of Intravascular Lithotripsy (IVL) to treat complex calcified cardiovascular disease, today reported financial results for the three months and full year ended December 31, 2019.
Recent Highlights
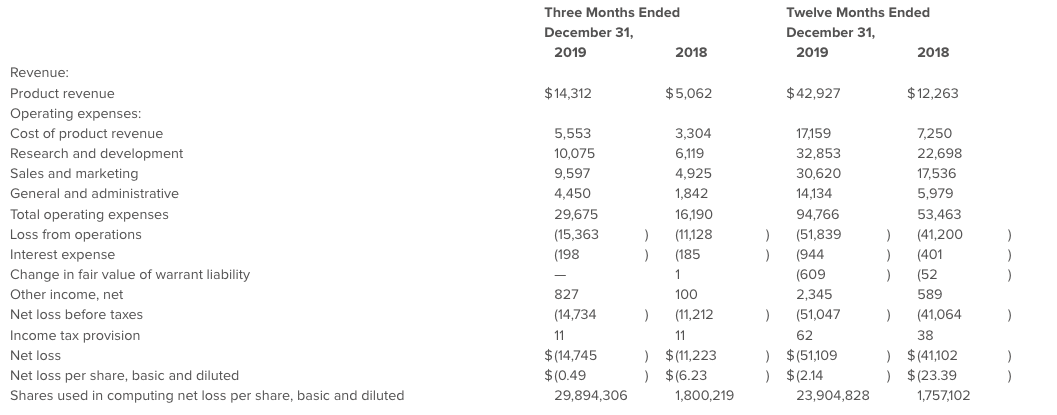
- Recognized revenue of $14.3 million for the fourth quarter and $42.9 million for the full year of 2019, representing increases of 183% and 250%, respectively, over the corresponding periods of 2018
- Commenced the full commercial launch of S4 Peripheral IVL Catheter in the U.S.
- Initiated the CAD IV study of IVL in Japan
- Ended the year with total enrollment of 346 patients in the CAD III IDE study in the U.S.
- Entered into a new sublease agreement – more than doubling the Company’s office space to approximately 85,200 square feet
- Completed an underwritten public offering raising $97 million in net proceeds
“I am encouraged by the progress we have made at Shockwave over the course of 2019. Our team improved their performance across the board – from clinical to sales to operations – as we all worked together to advance the use of IVL and to help change the way calcified cardiovascular disease is treated,” said Doug Godshall, President and Chief Executive Officer of Shockwave Medical. “The growing use of IVL across many different vessels has exceeded our expectations and I believe that Shockwave is well positioned to continue to positively impact our customers and their patients into the future.”
Fourth Quarter 2019 Financial Results
Revenue for the fourth quarter of 2019 was $14.3 million, an increase of $9.3 million, or 183%, compared to the fourth quarter of 2018. The growth was primarily driven by the expansion of the U.S. sales force and increased penetration in both U.S. and international markets.
Gross profit for the fourth quarter of 2019 was $8.8 million compared to $1.8 million for the fourth quarter of 2018. The gross margin percentage for the fourth quarter of 2019 increased to 61% compared to 35% in the fourth quarter of 2018, driven primarily by continued improvements in production processes and greater absorption of fixed costs by higher production.
Operating expenses were $24.1 million for the fourth quarter of 2019 compared to $12.9 million in the corresponding prior year period, an increase of 87%, primarily driven by sales force expansion and higher clinical costs from the CAD III IDE and CAD IV Japan studies.
Net loss was $14.7 million in the fourth quarter of 2019, as compared to $11.2 million in the corresponding period of the prior year. Net loss per share was $0.49 in the fourth quarter of 2019.
Full Year 2019 Financial Results
Revenue for full year 2019 was $42.9 million, an increase of $30.7 million, or 250%, compared to the full year of 2018. The growth was primarily driven by the expansion of the U.S. sales force and international distributor network.
Gross profit for the full year 2019 was $25.8 million compared to $5.0 million for the full year 2018. The gross margin percentage for the full year 2019 increased to 60% compared to 41% in the full year 2018, driven primarily by continued improvements in production processes and greater absorption of fixed costs by higher production.
Operating expenses were $77.6 million for the full year 2019 compared to $46.2 million in the prior year, an increase of 68%, primarily driven by sales force expansion and higher clinical costs from the CAD III IDE and CAD IV Japan studies.
Net loss was $51.1 million for full year 2019, as compared to $41.1 million in the prior year. Net loss per share was $2.14 for full year 2019.
Cash, cash equivalents and short-term investments totaled $195.3 million as of December 31, 2019.
2020 Financial Guidance
Shockwave Medical projects revenue for the full year 2020 to range from $74 million to $77 million, which would represent 72% to 79% growth over the company’s prior year revenue.
Conference Call
Shockwave Medical will host a conference call at 1:30 p.m. Pacific Time / 4:30 p.m. Eastern Time on Thursday, February 13, 2020 to discuss its fourth quarter and full year 2019 financial results. The call may be accessed through an operator by dialing (866) 795-9106 for domestic callers or (470) 495-9173 for international callers, using conference ID: 5764306. A live and archived webcast of the event will be available at https://ir.shockwavemedical.com/.
About Shockwave Medical, Inc.
Shockwave Medical is focused on developing and commercializing products intended to transform the way calcified cardiovascular disease is treated. The company aims to establish a new standard of care for medical device treatment of atherosclerotic cardiovascular disease through their differentiated and proprietary local delivery of sonic pressure waves for the treatment of calcified plaque, which they refer to as Intravascular Lithotripsy (IVL). IVL is a minimally invasive, easy-to-use and safe way to significantly improve patient outcomes. To view an animation of the IVL procedure and for more information, visit www.shockwavemedical.com.
Forward-Looking Statements
This press release contains statements relating to Shockwave’s expectations, projections, beliefs, and prospects (including statements regarding Shockwave’s financial and business outlook), which are “forward-looking statements” within the meaning of the federal securities laws and by their nature are uncertain. Words such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “plans,” and similar expressions are intended to identify forward-looking statements. Such forward-looking statements are not guarantees of future performance, and you are cautioned not to place undue reliance on these forward-looking statements. Our business and operations are subject to a variety of risks and uncertainties and, consequently, actual results may differ materially from those projected by any forward-looking statements. Factors that could cause actual results to differ from those projected include, but are not limited to: failure to achieve or sustain profitability; failure to effectively market existing products; failure to effectively introduce and market new products; delays in product introductions; significant competition; inability to further penetrate our current customer base and increase the frequency of use of our products by our customers; inability to achieve or maintain satisfactory pricing and margins; manufacturing difficulties; the inability to attain coverage and adequate reimbursement for procedures using our products; permanent write-downs or write-offs of our inventory; product defects or failures; unfavorable outcomes in clinical trials; inability to maintain our culture as we grow; fluctuations in foreign currency exchange rates; potential adverse regulatory actions; and potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make. These risks and uncertainties, as well as others, are discussed in greater detail in our filings with the Securities and Exchange Commission (SEC), including under the section entitled “Risk Factors” in our Quarterly Report on Form 10-Q for the nine months ended September 30, 2019 and in our prospectus dated November 14, 2019. There may be additional risks of which we are not presently aware or that we currently believe are immaterial which could have an adverse impact on our business. Any forward-looking statements are based on our current expectations, estimates and assumptions regarding future events and are applicable only as of the dates of such statements. We make no commitment to revise or update any forward-looking statements in order to reflect events or circumstances that may change.
Investor Contact:
Debbie Kaster, Gilmartin Group
investors@shockwavemedical.com