DISRUPT CAD III
Shockwave’s pivotal study demonstrating the safety, effectiveness and ease of use of Shockwave coronary Intravascular Lithotripsy (IVL).


Study leadership
This section contains attributions including profile pictures, titles, descriptions, and Twitter handles.
-
Jonathan Hill, MDCo-Principal Investigator, Consultant Cardiologist Kings College Hospital, London, UK
-
Dean Kereiakes, MD, FACC, FSCAICo-Principal Investigator, Medical Director The Christ Hospital Heart and Vascular Center, Cincinatti, OH
Statistics Callout
This section presents key statistical information with numbers and descriptions.
-
384Patients
-
47Sites
-
100%Severe Ca++
-
47.9mmCalcium length
-
98%Shockwave IVL crossing & therapy delivery
-
99%Stent delivery
-
0.3%Final major dissections
-
0.3%Final perforations
-
0.3%Abrupt closure
-
0%Slow flow/no reflow
-
11.9%Residual stenosis
-
1.7mmAcute gain
Key Findings
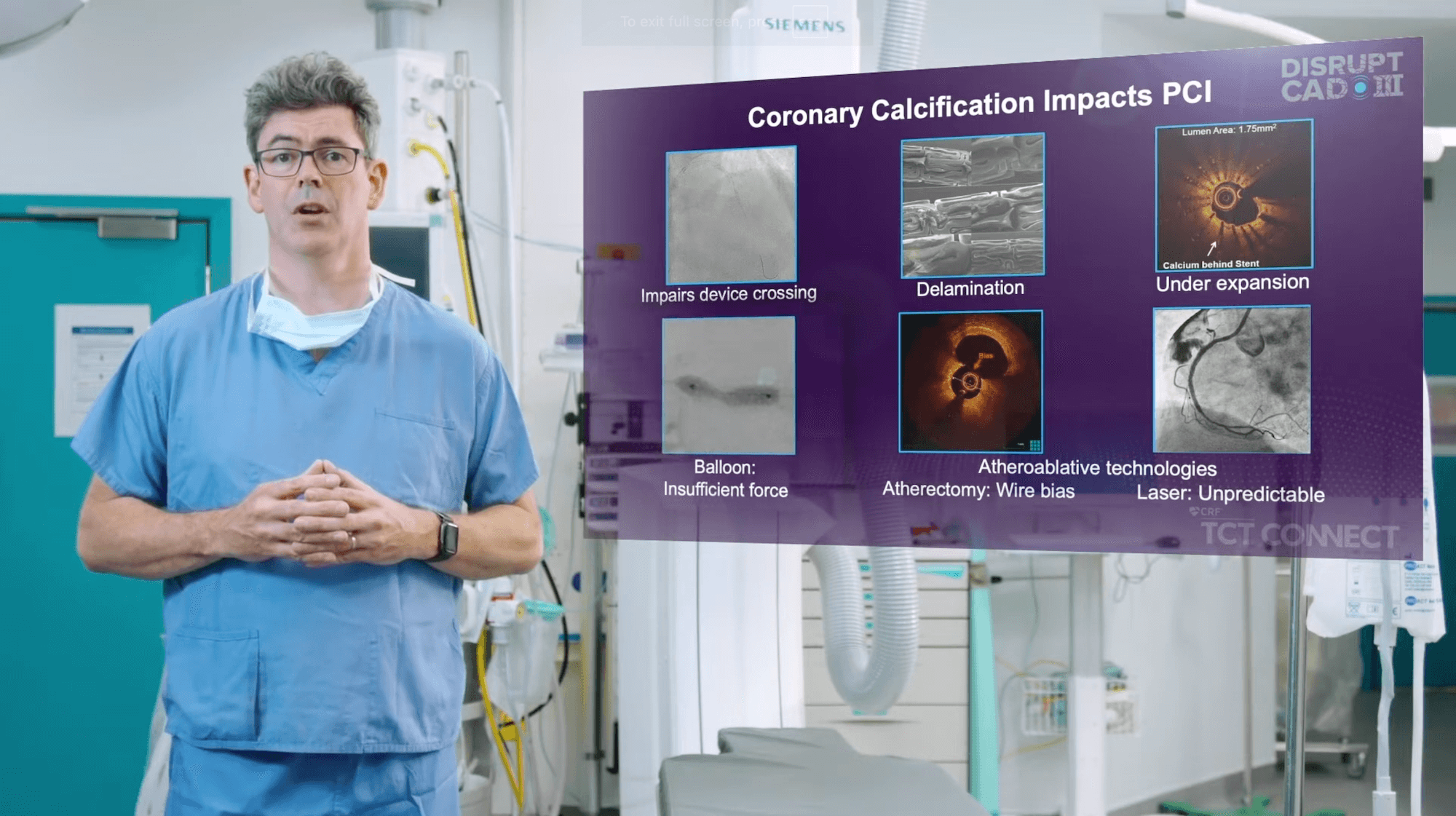
CAD III confirms Shockwave IVL safety with low rates of major peri-procedural clinical and angiographic complications, setting a new bar for safety in complex calcified coronary lesions.
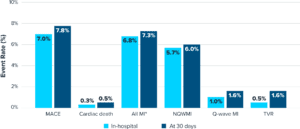
- 92.2% of patients were free from major adverse cardiovascular events (MACE) at 30 days, a composite of cardiac death (CD) (0.5%), myocardial infarction (MI) (7.3%), or target vessel revascularization (TVR) (1.6%)
- Primary MACE driver was in-hospital non-Q-wave MI (5.7%)
- Low risk of perforation (0.3%), major dissection (0.3%), abrupt closure (0.3%) and slow flow/no reflow (0.0%) at the end of procedure
CAD III showcases Shockwave IVL effectiveness with large lumen gains that facilitate stent delivery and optimize stent expansion.
- 92.4% procedural success rate, defined as successful stent delivery (99%) residual stenosis <50% (100%) and without in-hospital MACE (93%)
- Successful Shockwave IVL crossing and therapy delivery in 98% of lesions, correlating to 99% stent delivery
- 1.7 mm acute gain and 11.9% final in-stent residual stenosis
CAD III demonstrated Shockwave coronary IVL’s ease of use and quick learning curve to achieve consistently predictable outcomes.
- MACE, procedural success and device crossing success were similar between roll-in procedures (first case for each site) and procedures included in the pivotal analysis (taken from the DISRUPT CAD III publication)
Study Design and Patient Characteristics
Objective
Prospective, multicenter, single-arm global investigational device exemption (IDE) to evaluate the safety and effectiveness of Shockwave coronary IVL
Primary Safety Endpoint
Freedom from MACE (cardiac death, myocardial infarction or target vessel revascularization) at 30 days
Primary Effectiveness Endpoint
Successful stent delivery with residual stenosis <50% and without in-hospital MACE
Secondary Performance Endpoint
Clinical and angiographic success
Patient Characteristics Summary
- 71 years old
- 40% diabetes mellitus (DM)
- 3.0 mm right ventricular systolic dysfunction (RVD)
- 65% diameter stenosis
- 48 mm Ca++ length
- 26 mm lesion length
- 57% LAD, 13% LCS, 29% RCA, 1% LM
- 292 Ca++ arc at max Ca++ site
- 0.96 mm thick Ca++ at max Ca++ site
Accordion Section

*Final in-stent diameter stenosis of ≤30% achieved in 99.5% of patients
DISRUPT CAD III Resources
More DISRUPT CAD Studies
-
Single-arm, pre-market study demonstrating the safety and performance of Shockwave IVL in heavily calcified coronary lesions.Coronary IVL
-
European post-market study confirming DISRUPT CAD I results and showing strong safety and procedural success with Shockwave coronary IVL.Coronary IVL
-
Shockwave coronary study demonstrating the safety and effectiveness of Shockwave IVL within a Japanese patient population.Coronary IVL
Dr. Hill is a paid consultant for Shockwave Medical.