Dr. Michael Siah’s E8 Case
Case Courtesy of Dr. Michael Siah
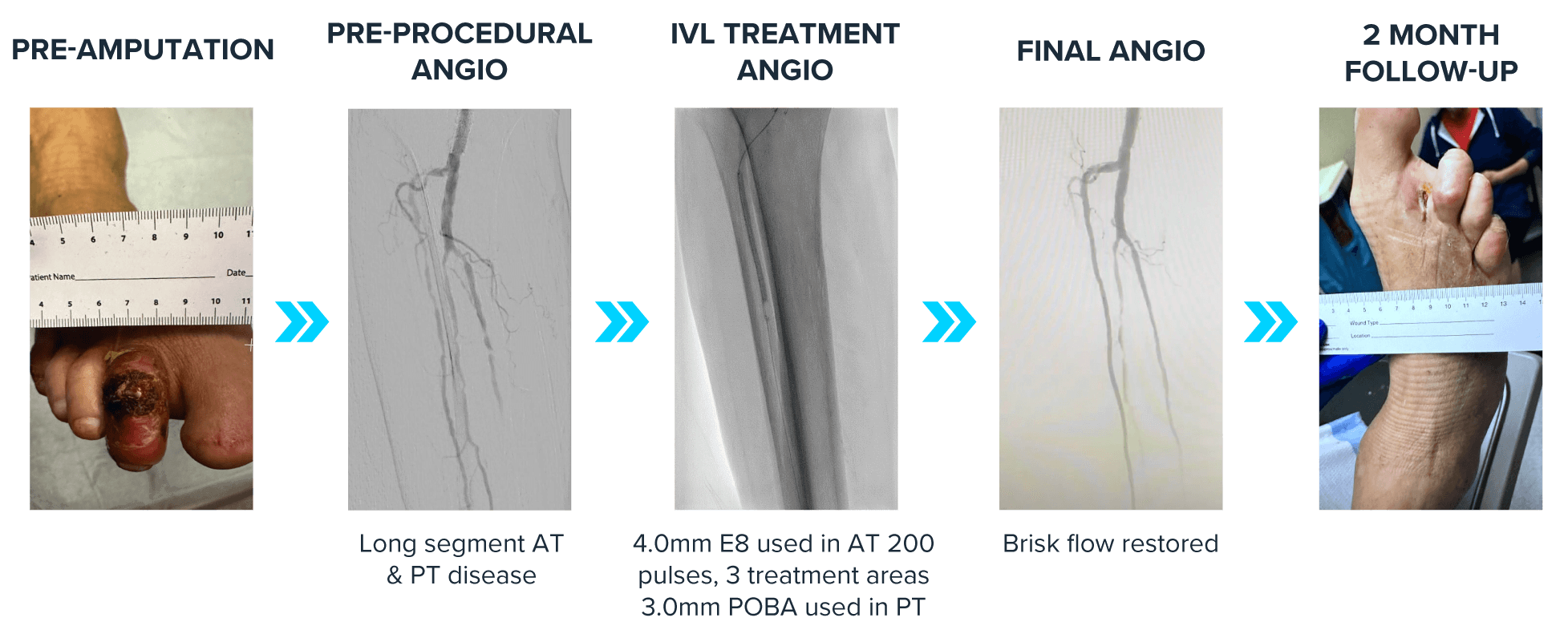
79M patient with CLTI and non-healing wound on his right second toe. His comorbidities include CAD, AF, DVT, CHF (EF 30%), and DM2. He previously had a CABG, and right popliteal angioplasty (2022). The patient underwent a second toe amputation prior to this revascularization. The patient was non-compressible and had an initial toe pressure of 15. The initial angiogram showed long segment AT disease and slow flow in the PT. Dr. Siah treated the AT with a Shockwave E8 4.0 mm, using 200 pulses over three treatment areas. The catheter was easily delivered and no pre-dilation was required. IVL was used as a stand-alone treatment in the AT and brisk flow was achieved with no complications. He then treated the focal PT stenosis with a 3.0 mm POBA. Post-procedure the patient’s toe pressure was 85. At two-month follow-up the patient’s wound has healed significantly and the AT remained open providing brisk flow to the foot. Shockwave E8 allowed for safe and effective treatment of long-segment AT disease in this patient with CLTI, ultimately supporting wound healing for this patient.
Dr. Michael Siah is a paid consultant of Shockwave Medical.
Peripheral IVL
Shockwave M5+, Shockwave M5, Shockwave S4, Shockwave L6 and Shockwave E8 Safety Information
In the United States: Rx only.
Indications for Use—The Shockwave Medical Intravascular Lithotripsy (IVL) System is intended for lithotripsy-enhanced balloon dilatation of lesions, including calcified lesions, in the peripheral vasculature, including the iliac, femoral, ilio-femoral, popliteal, infra-popliteal, and renal arteries. Not for use in the coronary, carotid or cerebral vasculature.
Contraindications—Do not use if unable to pass 0.014″ (M5, M5+, S4, E8) or 0.018″ (L6) guidewire across the lesion-Not intended for treatment of in-stent restenosis or in coronary, carotid, or cerebrovascular arteries.
Warnings—Only to be used by physicians who are familiar with interventional vascular procedures—Physicians must be trained prior to use of the device—Use the generator in accordance with recommended settings as stated in the Operator’s Manual.
Precautions—use only the recommended balloon inflation medium—Appropriate anticoagulant therapy should be administered by the physician—Decision regarding use of distal protection should be made based on physician assessment of treatment lesion morphology.
Adverse effects–Possible adverse effects consistent with standard angioplasty include–Access site complications–Allergy to contrast or blood thinner–Arterial bypass surgery—Bleeding complications—Death—Fracture of guidewire or device—Hypertension/Hypotension—Infection/sepsis—Placement of a stent—renal failure—Shock/pulmonary edema—target vessel stenosis or occlusion—Vascular complications. Risks unique to the device and its use—Allergy to catheter material(s)— Device malfunction or failure—Excess heat at target site.
Prior to use, please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions and adverse events. www.shockwavemedical.com/ifu



