Road to Relief: Effective Treatment of Refractory Angina Patients with Shockwave Reducer
At EuroPCR 2025, anchorperson James Spratt, spokesperson Felix Woitek and discussants Ranil de Silva, Mariusz Tomaniak and Tommaso Gori took the stage to present “Road to Relief: Effective Treatment of Refractory Angina Patients with Shockwave Reducer.”
Incidence & Impact of Refractory Angina
Watch this video in which Dr. de Silva (Royal Brompton Hospital, London) provides an overview of the definition, prevalence, challenges and impact of refractory angina on patients’ lives. Why is angina a problem? Why is angina misunderstood? These are just some of the questions Dr. de Silva tackles before exploring a real-world patient case.
Mechanism of Action (MOA)
Tune in to watch Prof. Spratt (St George’s Hospital, London) review the hypothesized MOA of the Shockwave Reducer device, first exploring how flow is maintained in a healthy heart versus a diseased heart, before diving into how Shockwave Reducer works to redistribute flow. Additionally, Dr. Spratt touches on the ORBITA-COSMIC clinical study, which provides further insights into Reducer’s MOA.
Reducer Implanting Procedure & Patient Follow Up
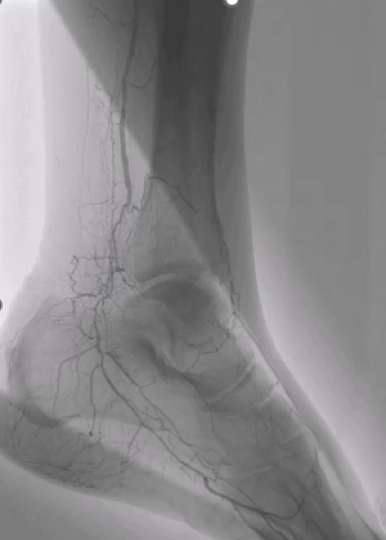
Join Prof. Tomaniak (Medical University of Warsaw, Poland) as he explores a real-world patient case example to demonstrate the four simple steps to implant the Shockwave Reducer – prepare, assess, deploy and retract. To conclude, Prof. Tomaniak emphasizes the importance to develop a successful referral network to increase the patient inflow to a Reducer program.
Clinical Data & Real-Life Results
Watch this video to see Prof. Gori (University Medical Centre of Mainz, Germany) review the comprehensive and compelling clinical data on Reducer and watch a patient testimonial from one of his patients to learn about the real-life impact of the Reducer.
Individual patient stories. Results may vary.
The physicians featured are paid consultants of Shockwave Medical.
Shockwave Reducer is commercially available in select European countries and has been implanted in over 3,500 patients. It is currently under clinical investigation in the U.S.
CAUTION: In the United States, Shockwave Reducer is an investigational device, limited by United States law to investigational use. Shockwave Reducer is subject of investigational testing and is being studied in the COSIRA-II trial in Canada. Shockwave Reducer is commercially available in certain countries outside the U.S. and Canada. Please contact your local representative for specific country availability. Prior to use, please reference the Instructions for Use for more information on warnings, precautions and adverse events: ifu.sw-reducer.com