DISRUPT PAD III Observational Study
The largest prospective real-world evidence for the treatment of complex, heavily calcified peripheral artery disease (PAD).


DISRUPT PAD III Observational Study (OS)
-
Objective
Assess real-world peri-procedural outcomes of Shockwave Intravascular Lithotripsy (IVL) for treatment of calcified, stenotic, peripheral arteries -
Design
Prospective, multicenter, single-blind, observational study -
Key inclusion
Rutherford classification 2-6. Moderate-severe calcification* ilio-femoral, femoral, popliteal and infrapopliteal arteries
*Presence of fluoroscopic evidence of calcification by PARC definition: 1) on parallel sides of the vessel and 2) extending > 50% the length of the lesion if lesion is ≥50 mm in length; or extending for minimum of 20 mm if lesion is <50 mm in length
Statistics Callout
This section presents key statistical information with numbers and descriptions.
-
1,373Patients
-
1,677Lesions
-
30Sites
-
3Countries
Armstrong E, VIVA Late Breaking Clinical Trial 2022
Complex Real-World Patients
Statistics Callout
This section presents key statistical information with numbers and descriptions.
-
1,373Patients
-
36%Critical Limb Ischemia (CLI) patients
-
56%Diabetes Mellitus
-
27%Renal Insufficiency
Challenging Lesions
Statistics Callout
This section presents key statistical information with numbers and descriptions.
-
1,531Lesions
-
90%Moderate-severe calcium*
-
115mmAverage calcified length
-
31%Chronic Total Occlusions (CTOs)
*PARC Definition
Armstrong E, VIVA Late Breaking Clinical Trial 2022
Predictable Outcomes in Challenging Situations
Predictably consistent results across vessel beds, challenging lesions and complex patients
MAJORITY OF STENOSIS REDUCTION FROM IVL TREATMENT
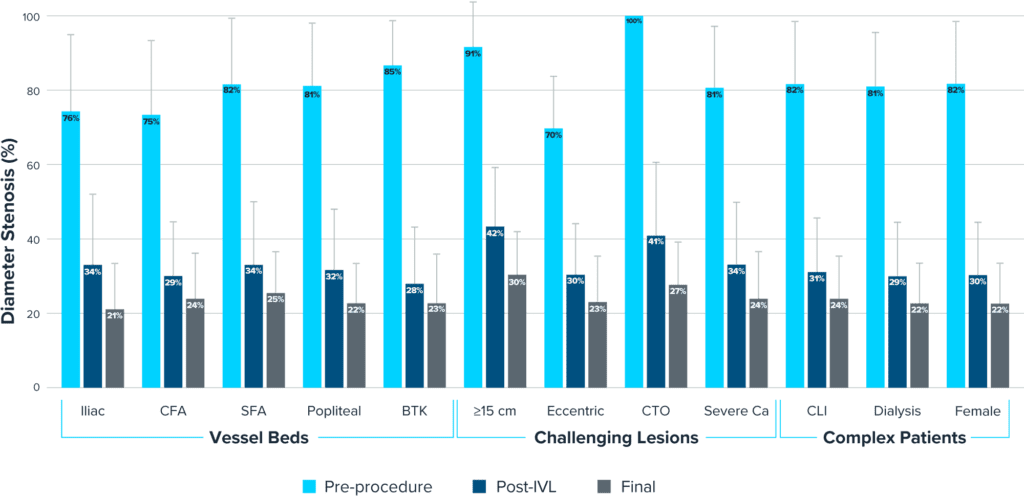
Armstrong E, Late Breaking Clinical Trial and Shockwave-Sponsored Symposium, VIVA 2022
Shockwave IVL Procedural Insights
Use of adjunctive technology was at the operator’s discretion; use of embolic protection was less when Shockwave IVL was used as the only calcium modification tool; Shockwave IVL saw better results with appropriate Shockwave IVL sizing.
When Shockwave IVL was used as the only calcium modification therapy*, there was less use of embolic protection.

Per a multivariable analysis, proper Shockwave IVL balloon sizing (≥ 1:1)** is an independent predictor of improved stenosis reduction but not a predictor of complications.

*Ca modifying therapy: atherectomy and/or scoring/cutting balloon
**PAD OS data analysis is consistent with prior PAD II data analysis and continues to support 1.1:1 sizing in the product instructions for use (IFU)
Armstrong E, VIVA Late Breaking Clinical Trial 2022
Real-World Outcomes Consistent with Randomized Trial
Shockwave IVL safely and effectively modifies calcium across multiple vessel beds.
FINAL ANGIOGRAPHIC COMPLICATIONS (CORE LAB)
| DISRUPT PAD III RCT1 | DISRUPT PAD III OS2 | |
| N | 153 | 1,367 |
| Vessels | Superficial femoral artery (SFA)/popliteal | Iliac, common femoral artery (CFA), SFA/popliteal, infrapopliteal |
| Dissection (Type D-F) | 0% | 0.7% |
| Perforation | 0% | 0.2% |
| Embolization | 0% | 0% |
| Slow flow/no reflow | 0% | 0% |
| Abrupt closer | 0% | 0% |
| Thrombus | 0% | 0% |
Proven Effective Calcium Modification
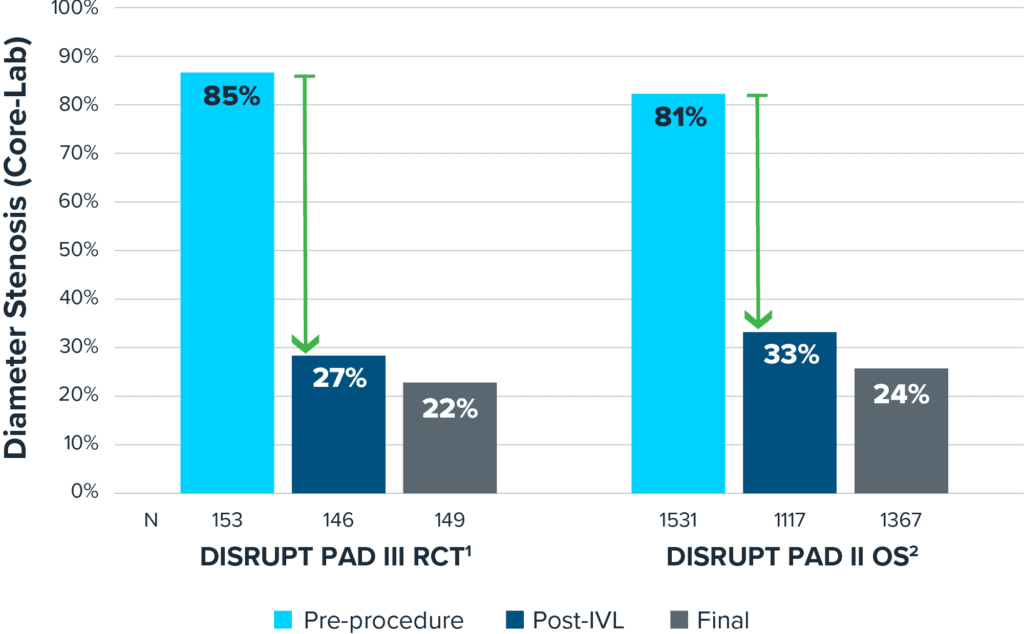
1: Tepe et al, J Am Coll Cardiol Intv 2021
2: E. Armstrong, VIVA Late Breaking Clinical Trial 2022
More Shockwave Peripheral IVL Studies
-
Global prospective, multi-center, single-arm study assessing the safety and effectiveness of Shockwave peripheral IVL in treating long, calcified BTK lesions.Peripheral IVL
-
Core lab adjudicated, long-term, multi-center study exclusively enrolling heavily calcified lesions building upon DISRUPT PAD I.Peripheral IVL
-
The largest-ever randomized clinical study of Shockwave peripheral IVL treatment in severely calcified peripheral lesions, out to 24 months.Peripheral IVL