Following its presentation as a late-breaking clinical at TCT Connect and simultaneous publication in the Journal of the American College of Cardiology (JACC) we sat down with Dr. Jonathan Hill, Consultant Cardiologist at the Royal Brompton Hospital in London, to get his perspective on the results, how they build on DISRUPT CAD I and II outcomes, and where they fit into clinician’s daily decision-making process when treating calcified lesions. We hope you enjoy his perspective.
What does DISRUPT CAD III add to the existing results from DISRUPT CAD I and DISRUPT CAD II?
Dr. Hill: CAD III is the culmination of four years of research in over 600 patients that has spanned three studies. Its results are noteworthy in that it’s the first study that has been appropriately powered to evaluate the safety and effectiveness of IVL, which is important when evaluating low frequency angiographic complications, such as perforations and dissections, as well as clinical events, such as MI and TVR. It also is the first IVL study to prospectively look at IVL’s impact on heart rhythm, and follow patients for two years. Finally, we studied more patients with OCT than CAD I and II combined. For all of these reasons, its results have unveiled a significant amount of new insights about the therapy.
How would you summarize the key findings from DISRUPT CAD III?
Dr. Hill: There are four key findings from my perspective. First, CAD III successfully met both its primary safety and effectiveness endpoints, despite having one of the most challenging cohort of calcified lesions ever studied. Second, coronary IVL prior to DES implantation was well tolerated with a low rate of major peri-procedural clinical and angiographic complications, similar to what we found in CAD I and II. Third, IVL-induced ventricular capture was common, but was transient and benign with no clinical sequelae in any patient. And lastly, although this study represents the initial coronary IVL experience for U.S. operators, similar high procedural success and device crossing success, as well as low MACE and angiographic complications were achieved in both the initial roll-in cases and the patients included in the ITT analysis, demonstrating the relative ease of use of IVL for the first time in a study.
Why do you say that this was one of the most challenging cohorts of calcified lesions ever studied?
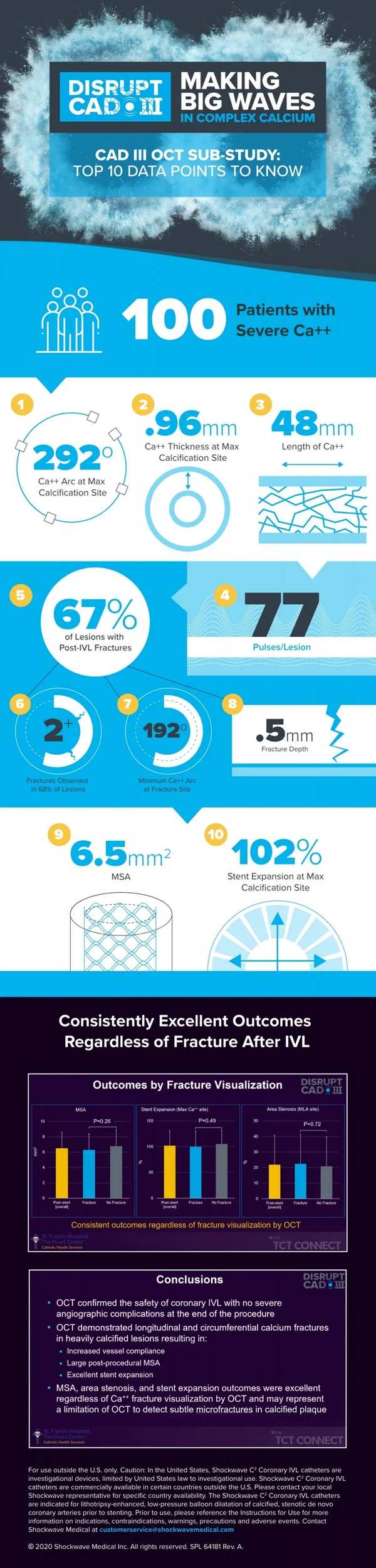
Dr. Hill: Following the ORBIT II design as our predicate study, the inclusion criteria for CAD III was designed to focus on finding the most severely calcified patients, only including patients with angiographic evidence of calcium on both sides of the artery, or more than 270 degrees of calcium under intravascular imaging. We were successful to that end, enrolling 100% of patients with severe calcium. The average calcium lesion length was very long at 47.9 mm, and the average calcium arc was 292.5 degrees with a thickness of 0.96 mm at the site of maximum calcification as measured by OCT. If you’re familiar with the rule of 5s with OCT, you’ll know that these are the most challenging type of calcified lesions to treat: thick, circumferential and diffuse calcium.
Were there any surprises or unexpected findings from DISRUPT CAD III?
Dr. Hill: One of the current limitations of the technology has been its higher crossing profile when compared to a normal NC balloon. However, despite the marked severity of the calcified lesions treated, IVL was able to cross and deliver therapy in 98.2% of lesions, or 377 of 384 total patients, which then lead to a 99% rate of stent deliverability. I was surprised to see such a high crossing rate, especially considering that this was U.S. operators first use of the technology.
Another unexpected finding was related to fracture evidence under OCT. Interestingly, OCT showed excellent stent expansion in those lesions with and without calcium fractures identified by OCT despite the marked severity of the calcified lesions treated, meaning that IVL led to improved vessel compliance and favorable stent expansion even without demonstrable calcium fracture by OCT. I think that finding will surprise my fellow colleagues who use OCT regularly and have trained themselves to look for fractures to know if they have delivered enough energy to adequately prepare the artery for stent implantation. This raises the prospect of IVL induced microfractures not visible by OCT as an additional mechanism of increasing vessel compliance.
You mentioned IVL-induced ventricular capture was investigated prospectively in DISRUPT CAD III for the first time – what were the conclusions regarding this topic?
Dr. Hill: As many have seen in their own use with the technology, as well as what has been reported previously about IVL-induced ventricular capture in the EuroIntervention publication, CAD III found that this phenomenon was common, occurring in just over 40% of cases. Importantly, no serious adverse clinical events occurred as a result of IVL-induced capture. It was significantly more common if the pre-procedure heart rate was lower, particularly by multivariate analysis less than 60BPM. The drop in systolic blood pressure was more frequent, but not more severe, based on this analysis. And most important this was a transient phenomenon which resolved once IVL delivery stopped without significant sequalae.
What are your main takeaways from the OCT sub-study?
Dr. Hill: I think my main takeaways from the OCT sub-study would be that with 100 OCT patients studied, we have the largest analysis yet that provides us with new evidence that confirms and extends prior findings on IVL’s unique mechanism of action that enables optimal stent delivery, expansion and apposition. A few of the stats that I thought were interesting were the final MSA of 6.5 mm, which is a great outcome, the final stent expansion of 102% at the site of maximum calcification (where the calcium angle was 292° and 0.96 mm thick on average), and that post-IVL calcium fractures were observed in multiple longitudinal planes in 67% of lesions.
Looking specifically at the fractures, four correlative findings were that the minimum calcium angle that resulted in calcium fracture was 192° and the result already mentioned that regardless of calcium fracture identification by OCT, there were no differences between lesions with or without fractures in relation to the MSA, final area stenosis, and stent expansion. The fractures were also an average depth of 0.5 mm deep and 0.5 mm wide, which expanded to 1.3 mm after stent implantation.
How does DISRUPT CAD III change your use of IVL or your daily practice of calcium management?
Dr. Hill: I think there are several practice-changing finds for operators across Europe who have been using the technology for some time now. First, we know what to expect now with IVL-induced capture, including how often it occurs, in which scenarios and what impact it has on the patients so that we can mitigate it successfully. Two, when looking for post-IVL fractures for those who use OCT, the CAD III data would suggest that evidence of fracture is not a necessary indicator of adequate lesion preparation with IVL.
For those individuals who do not use intravascular imaging in everyday practice, this data shows that overall balloon expansion under angiography may be an alternative way to determine if the calcium has been modified adequately and that the vessel compliance has changed sufficiently for DES implantation.
Where do we go from here in regard to clinical evidence with IVL?
Dr. Hill: The study of IVL across CAD I, CAD II and CAD III provides a robust clinical data set of over 600 patients, and from the CAD III study, we have identified additional areas of interest beyond what was observed in the initial studies. First, I think it’s important to note that the JACC publication is the 30-day data, and these patients will be followed out to two years, which will be important as this will be the longest and most rigorous follow-up conducted on coronary IVL patients to date. Second, the fact that MSA, area stenosis, and stent expansion outcomes were excellent regardless of calcium fracture visualization by OCT is a novel finding and challenges the accepted practice of visual confirmation when using calcium modification tools. It may represent OCT’s limitation to detect subtle micro-fractures or out-of-plane fractures in calcified plaque, but this is just a hypothesis and we need to further assess. Third, while this patient population consisted of mostly lesions with circumferential calcium, further assessment of the OCT patients treated within the DISRUPT CAD program, which should be nearly 250 patients, will provide more insights regarding the similar performance in concentric & eccentric lesions that was previously found in the CAD II angiography-based study. And finally, addressing the major limitations of nearly all calcium modifications algorithms that are coming to light (with none based on data), randomized data across multiple calcium tools may be warranted. I have no doubt that RCTs will be done that include IVL and other calcium tools; in fact there are several smaller RCT – some investigator-sponsored and others independent – that have been initiated, and I look forward to their outcomes.
Dr. Jonathan Hill is a paid consultant of Shockwave Medical.
Coronary Important Safety Information:
In the United States: Rx only.
Indications for Use—The Shockwave Intravascular Lithotripsy (IVL) System with the Shockwave C2 Coronary IVL Catheter is indicated for lithotripsy-enabled, low-pressure balloon dilatation of severely calcified, stenotic de novo coronary arteries prior to stenting.
Contraindications—The Shockwave C2 Coronary IVL System is contraindicated for the following: This device is not intended for stent delivery. This device is not intended for use in carotid or cerebrovascular arteries.
Warnings— Use the IVL Generator in accordance with recommended settings as stated in the Operator’s Manual. The risk of a dissection or perforation is increased in severely calcified lesions undergoing percutaneous treatment, including IVL. Appropriate provisional interventions should be readily available. Balloon loss of pressure was associated with a numerical increase in dissection which was not statistically significant and was not associated with MACE. Analysis indicates calcium length is a predictor of dissection and balloon loss of pressure. IVL generates mechanical pulses which may cause atrial or ventricular capture in bradycardic patients. In patients with implantable pacemakers and defibrillators, the asynchronous capture may interact with the sensing capabilities. Monitoring of the electrocardiographic rhythm and continuous arterial pressure during IVL treatment is required. In the event of clinically significant hemodynamic effects, temporarily cease delivery of IVL therapy.
Precautions— Only to be used by physicians trained in angiography and intravascular coronary procedures. Use only the recommended balloon inflation medium. Hydrophilic coating to be wet only with normal saline or water and care must be taken with sharp objects to avoid damage to the hydrophilic coating. Appropriate anticoagulant therapy should be administered by the physician. Precaution should be taken when treating patients with previous stenting within 5mm of target lesion.
Potential adverse effects consistent with standard based cardiac interventions include– Abrupt vessel closure – Allergic reaction to contrast medium, anticoagulant and/or antithrombotic therapy-Aneurysm-Arrhythmia-Arteriovenous fistula-Bleeding complications-Cardiac tamponade or pericardial effusion-Cardiopulmonary arrest-Cerebrovascular accident (CVA)-Coronary artery/vessel occlusion, perforation, rupture or dissection-Coronary artery spasm-Death-Emboli (air, tissue, thrombus or atherosclerotic emboli)-Emergency or non-emergency coronary artery bypass surgery-Emergency or non-emergency percutaneous coronary intervention-Entry site complications-Fracture of the guide wire or failure/malfunction of any component of the device that may or may not lead to device embolism, dissection, serious injury or surgical intervention-Hematoma at the vascular access site(s)-Hemorrhage-Hypertension/Hypotension-Infection/sepsis/fever-Myocardial Infarction-Myocardial Ischemia or unstable angina-Pain-Peripheral Ischemia-Pseudoaneurysm-Renal failure/insufficiency-Restenosis of the treated coronary artery leading to revascularization-Shock/pulmonary edema-Slow flow, no reflow, or abrupt closure of coronary artery-Stroke-Thrombus-Vessel closure, abrupt-Vessel injury requiring surgical repair-Vessel dissection, perforation, rupture, or spasm. Risks identified as related to the device and its use: Allergic/immunologic reaction to the catheter material(s) or coating-Device malfunction, failure, or balloon loss of pressure leading to device embolism, dissection, serious injury or surgical intervention-Atrial or ventricular extrasystole-Atrial or ventricular capture.
Prior to use, please reference the Instructions for Use for more information on warnings, precautions and adverse events. https://shockwavemedical.com/IFU
Please contact your local Shockwave representative for specific country availability and refer to the Shockwave C2 instructions for use containing important safety information.