The CathPCI Registry Post-Approval Study (PAS) aimed to understand the utilization and in-hospital outcomes of the Shockwave coronary intravascular lithotripsy (IVL) system in a real-world environment following its regulatory approval study. Throughout the study, a considerable number of patients with acute coronary syndrome (ACS) received IVL who were not included in the original regulatory studies, so the study investigators decided to do a post-hoc safety analysis on these patients.
In this Q&A, we invited Dr. Dean Kereiakes, Christ Hospital Heart and Vascular Institute, to share his interpretation of the study’s conclusions and the data he presented at SCAI 2024, as well as provide insight as to how he believes the findings may impact the daily practice of physicians treating calcified lesions in these additionally complex patients.
What is the significance of coronary artery calcification for patients undergoing PCI?
Dr. Kereiakes: Moderate to severe coronary artery calcification may be present in 25 to 30%1 of patients undergoing PCI, and it can significantly impact the outcomes by impeding optimal stent deployment through asymmetry, apposition and expansion, and coronary edge dissection. If coronary artery calcium is not adequately modified, MACE events increase peri-procedurally and throughout long term follow up (out to 10+ years).
What prompted the analysis of the Acute Coronary Syndrome subgroup?
Dr. Kereiakes: An analysis was conducted of 18,893 PCI procedures that utilized the Shockwave C2 coronary iVL technology in a real-world environment looking at data from the ACC NCDR Cath PCI registry to better understand the utilization and in-hospital outcomes. Of the 18,893 patients, only 6% satisfied the inclusion criteria of the PAS, meaning that 94% of the cohort did not meet the inclusion/exclusion criteria for the PAS and previously conducted DISRUPT CAD studies. The acute coronary syndrome sub-group made up 36% of the non-PAS group.
How has IVL provided an opportunity for challenging patient subsets?
Dr. Kereiakes: Intravascular lithotripsy has demonstrated safety based on the Cath PCI data, and based on the DISRUPT CAD series of trials, IVL has also demonstrated efficacy in modifying calcium. One could argue that’s a different population of patients, but the severity of calcium in DISRUPT CAD III, for example, was worse than other IDE trials, and IVL was very effective – minimum stent areas (MSAs) > 6 mm2 and stent expansion > 100%, regardless of the arc of calcium, even stratified by quartiles of calcium arc up to 360 degrees.
Tell us about the patient characteristics of the ACS subgroup?
Dr. Kereiakes: These patients are complex; they include multiple lesions, acute coronary syndrome, etc. The average age was 73, 67% were male, 10% of these patients were on hemodialysis for kidney replacement, 55% had diabetes, and 93% had hypertension. The average lesion length was 32 mm. Severe calcification (site determined, not core lab adjudicated) was 57%, and almost 80% of these patients had complex lesion morphology.
What were the outcomes for the ACS patients that were treated with IVL?
Dr. Kereiakes: The most common procedure performed was IVL + stent placement (69.4%), followed by IVL + stent + atherectomy (18.1%), and with respect to intra-procedure severe angiographic complications, coronary perforation in the ACS population was observed in 0.6%, and complex coronary artery dissection was observed in 0.8%. All data were site-reported, not independently adjudicated or assessed by a core lab, and predominantly comprised of in-hospital outcomes.
How was the bedside risk score applied and why is it an important metric when evaluating the use of technology?
Dr. Kereiakes: The bedside risk score, derived from the ACC NCDR Cath PCI database, is a validated model for predicting in-hospital mortality.2 With IVL, we are seeing in-hospital mortality rates that are in line with what we would predict.
The CATH PCI data analysis includes both STEMI and NSTEMI ACS patients. How should physicians approach these groups of patients when there’s calcium present?
Dr. Kereiakes: Anytime thrombus is present, it complicates the issue. Calcium is one thing, but calcium and thrombus makes it even more complex. It’s been my experience that the chance of slow flow and no reflow are increased, particularly if using atheroablative technologies, so having the ability to modify calcium and to optimize stent deployment without using atheroablative technologies is a benefit and is a step forward in our portfolio of tools to intervene in ACS patients.
How does the Cath PCI data inform calcium modification strategy with ACS patients?
Dr. Kereiakes: First and foremost, the Cath PCI data supports the safety of this technology. For many of these patients, it was the center’s first opportunity to utilize the technology, and the observed mortality in the PAS, Non-PAS and the ACS subsets of the Cath PCI data is in line with what would be predicted based on risk scoring. This is a complex group of patients and the safety as reflected by in-hospital mortality is in line with what would be predicted based on the complexity of any of those groups – ACS, NSTEMI, or STEMI.
How should physicians use this new data?
Dr. Kereiakes: Physicians can use this data both for comfort and for confidence for themselves and when discussing it with patients. Based on data from a large population of patients, we know that IVL is a safe technique, and based on prior Shockwave-sponsored studies that excluded the ACS patient population, it’s been shown to very effectively modify calcium and optimize stent deployment.
What are your main takeaways from this analysis?
Dr. Kereiakes: In the real-world more complex patients with complex lesions, the safety of IVL was consistent with prior clinical trials. ACS represents over 30% of all coronary IVL cases, and the observed in-hospital mortality rates were in line with the predicted risk.
1: Genereux P et al. JACC 2014; 63:1845-1854.
2: Castro-Dominguez Y et al. JACC 2021; 78:216-229.
IVL efficacy in an ACS subpopulation has not been studied or proven in Shockwave sponsored studies.
Views expressed are those of the author and not necessarily those of Shockwave Medical. Dr. Dean Kereiakes is a paid consultant for Shockwave Medical. See important safety information below.
Coronary IVL
Shockwave C2 and Shockwave C2+ Safety Information
In the United States: Rx only.
Indications for Use – The Shockwave Intravascular Lithotripsy (IVL) System with the Shockwave C2 and C2+ Coronary IVL Catheter is indicated for lithotripsy-enabled, low-pressure balloon dilatation of severely calcified, stenotic de novo coronary arteries prior to stenting.
Contraindications – The Shockwave C2 and C2+ Coronary IVL System is contraindicated for the following: This device is not intended for stent delivery. This device is not intended for use in carotid or cerebrovascular arteries.
Warnings – Use the IVL Generator in accordance with recommended settings as stated in the Operator’s Manual. The risk of a dissection or perforation is increased in severely calcified lesions undergoing percutaneous treatment, including IVL. Appropriate provisional interventions should be readily available. Balloon loss of pressure was associated with a numerical increase in dissection which was not statistically significant and was not associated with MACE. Analysis indicates calcium length is a predictor of dissection and balloon loss of pressure. IVL generates mechanical pulses which may cause atrial or ventricular capture in bradycardic patients. In patients with implantable pacemakers and defibrillators, the asynchronous capture may interact with the sensing capabilities. Monitoring of the electrocardiographic rhythm and continuous arterial pressure during IVL treatment is required. In the event of clinically significant hemodynamic effects, temporarily cease delivery of IVL therapy.
Precautions – Only to be used by physicians trained in angiography and intravascular coronary procedures. Use only the recommended balloon inflation medium. Hydrophilic coating to be wet only with normal saline or water and care must be taken with sharp objects to avoid damage to the hydrophilic coating. Appropriate anticoagulant therapy should be administered by the physician. Precaution should be taken when treating patients with previous stenting within 5mm of target lesion.
Potential adverse effects consistent with standard based cardiac interventions include- Abrupt vessel closure – Allergic reaction to contrast medium, anticoagulant and/or antithrombotic therapy-Aneurysm-Arrhythmia-Arteriovenous fistula-Bleeding complications-Cardiac tamponade or pericardial effusion-Cardiopulmonary arrest-Cerebrovascular accident (CVA)-Coronary artery/vessel occlusion, perforation, rupture or dissection-Coronary artery spasm-Death-Emboli (air, tissue, thrombus or atherosclerotic emboli)-Emergency or nonemergency coronary artery bypass surgery-Emergency or nonemergency percutaneous coronary intervention-Entry site complications-Fracture of the guide wire or failure/malfunction of any component of the device that may or may not lead to device embolism, dissection, serious injury or surgical intervention-Hematoma at the vascular access site(s)- Hemorrhage-Hypertension/Hypotension-Infection/sepsis/fever-Myocardial Infarction-Myocardial Ischemia or unstable angina-Pain-Peripheral Ischemia-Pseudoaneurysm-Renal failure/insufficiency-Restenosis of the treated coronary artery leading to revascularization-Shock/pulmonary edema-Slow flow, no reflow, or abrupt closure of coronary artery-Stroke Thrombus-Vessel closure, abrupt-Vessel injury requiring surgical repair-Vessel dissection, perforation, rupture, or spasm.
Risks identified as related to the device and its use: Allergic/immunologic reaction to the catheter material(s) or coating-Device malfunction, failure, or balloon loss of pressure leading to device embolism, dissection, serious injury or surgical intervention-Atrial or ventricular extrasystole-Atrial or ventricular capture.
Prior to use, please reference the Instructions for Use for more information on indications, contraindications, warnings, precautions and adverse events. www.shockwavemedical.com/IFU.








 results of the EMPOWER CAD which is designed to prospectively study IVL outcomes within female patients. I suspect the results of EMPOWER CAD will confirm the existing, positive results from the retrospective analysis.
results of the EMPOWER CAD which is designed to prospectively study IVL outcomes within female patients. I suspect the results of EMPOWER CAD will confirm the existing, positive results from the retrospective analysis. Though sizing 1:1 to the healthy RVD may be a customary approach seen in other devices like traditional balloon angioplasty, peripheral IVL is an exception to the rule. It’s recommended to oversize peripheral IVL by 10% (a ratio of 1.1:1) to the healthy RVD because it achieves better and sustained wall apposition, which leads to more efficient energy transfer from the IVL device.1, 2, 3
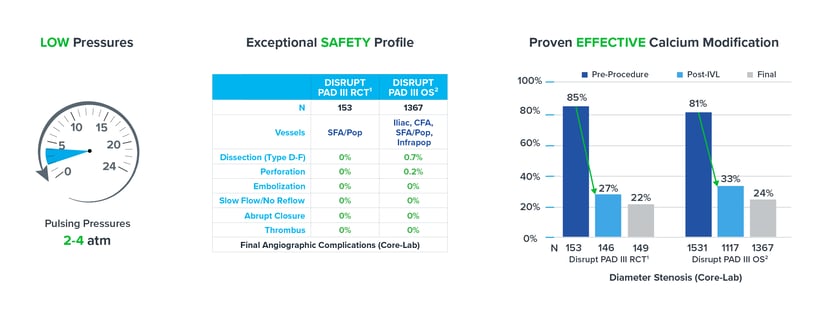
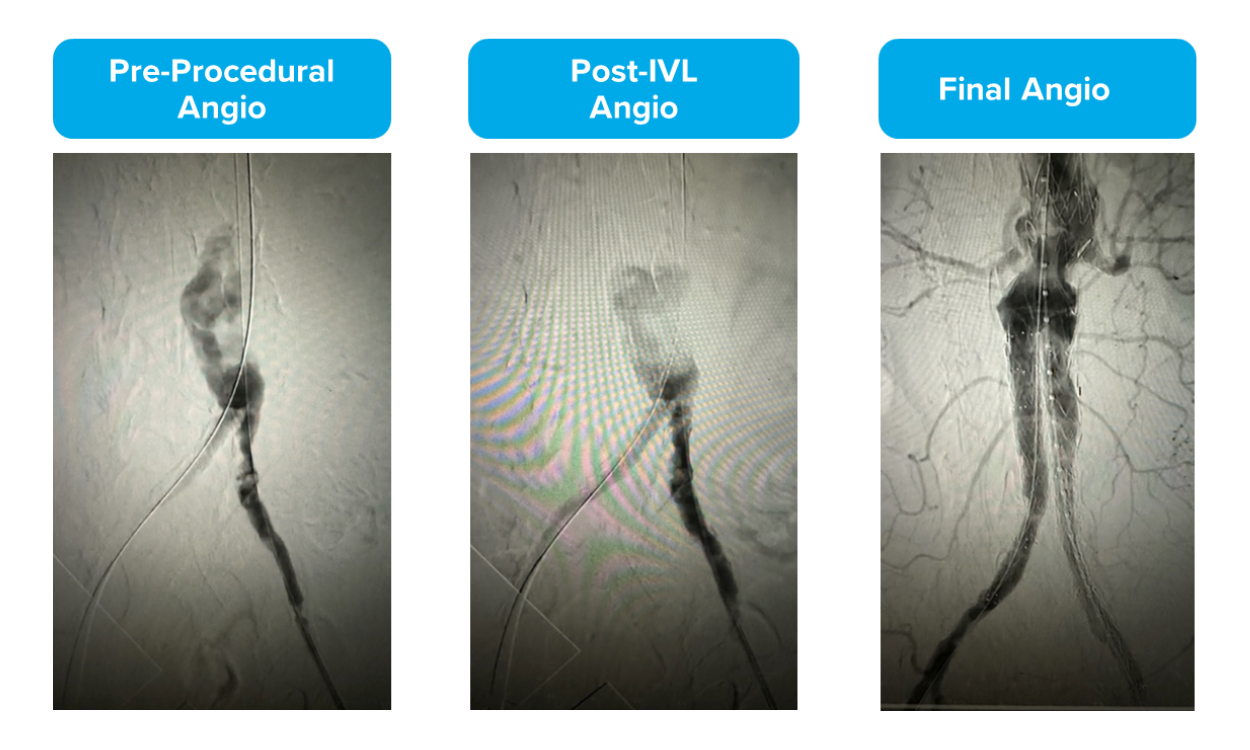
Though sizing 1:1 to the healthy RVD may be a customary approach seen in other devices like traditional balloon angioplasty, peripheral IVL is an exception to the rule. It’s recommended to oversize peripheral IVL by 10% (a ratio of 1.1:1) to the healthy RVD because it achieves better and sustained wall apposition, which leads to more efficient energy transfer from the IVL device.1, 2, 3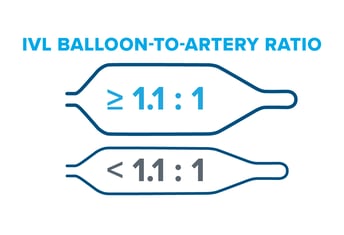 Evidence from the DISRUPT PAD clinical program (DISRUPT PAD II and DISRUPT PAD III Observational Study) shows that oversizing results in improved outcomes in terms of stenosis reduction and patency, all achieved with ultra-low treatment pressures (2-4 atm) and minimal complications, without compromising outcomes.
Evidence from the DISRUPT PAD clinical program (DISRUPT PAD II and DISRUPT PAD III Observational Study) shows that oversizing results in improved outcomes in terms of stenosis reduction and patency, all achieved with ultra-low treatment pressures (2-4 atm) and minimal complications, without compromising outcomes.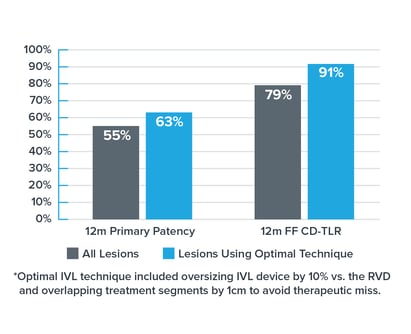 Improved Patency
Improved Patency