Initial Longer-term Outcomes Under Imaging with Coronary IVL
Intravascular lithotripsy (IVL) keeps making waves in the clinical community… a new publication has just been added to the already solid body of data supporting coronary IVL’s safety and efficacy: “Mid-Term Angiographic and Intracoronary Imaging Results Following Intracoronary Lithotripsy in Calcified Coronary Artery Disease: Results From Two Tertiary Referral Centres” was just published in Cardiovascular Revascularization Medicine journal. This recent study dives into mid-term angiographic and intracoronary imaging results post IVL usage, showing durable results with preserved stent parameters following IVL.
We have asked Dr. Angela McInerney, lead author of this publication, to give us a quick snapshot of the study, its key findings and conclusion. Keep on reading to find out more!
What was the aim of this study?
IVL has been in use in our institution since 2018 and our experience has been that the acute results were very successful with significant calcium fracture and adequate stent expansion. However, we did not have any data on the longer term results especially vascular healing following the use of this technology. For that reason, we decided to perform angiographic and intracoronary imaging follow up of our patients treated with IVL.
How do you summarize the study and its key findings and implications?
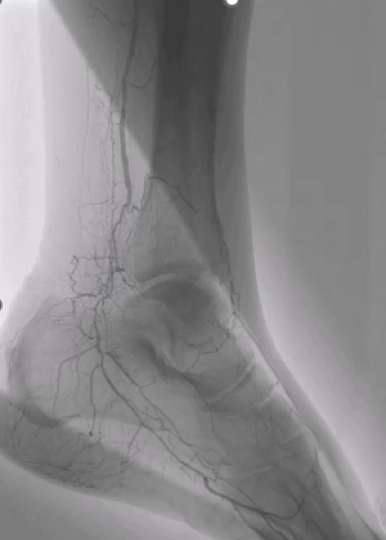
In this study, patients who underwent IVL for the treatment of calcified coronary artery disease were invited for repeat angiography with a subgroup also undergoing optical coherence tomography. In total 20 patients participated from two tertiary referral centres.
At the index IVL procedure, calcium fracture by OCT was seen in 89% of cases and median stent expansion was >90%. Follow up angiography was at a median of 22 months. A binary restenosis rate of 10% was found (2 patients) with only one needing treatment (second patient had a negative physiological assessment).
On paired OCT analysis there was no difference in stent expansion, minimum stent area and minimum lumen area from index to follow up procedure. Neointimal assessment by OCT demonstrated a homogenous neointima with a high backscatter representing health neointimal growth.
These result suggest durable results with preserved stent parameters following IVL at mid-term follow up with appropriate vascular healing.
Why is it important to study the healing patterns after IVL treatment?
IVL is a newly available calcium modification technique. Acutely, IVL results in deep fissuring and fracturing of the calcium allowing stent expansion. How these fractures may heal following stenting was heretofore unknown. Exuberant healing could result in excessive instent restenosis, while lack of stent strut coverage could also increase the risk of stent thrombosis. Understanding the vascular healing following IVL is therefore an important research question which we aimed to answer in this study.
What additional insights were you able to conclude out of the OCT substudy?
The OCT substudy demonstrated preserved stent parameters from index procedure to follow up. Parameters assessed included stent expansion and minimum stent area which are two of the most important parameters for predicting stent failure. The paired OCT analysis demonstrated stent expansion of >85% both at index procedure and follow up. Additionally, the OCT findings demonstrated healthy neointimal coverage of stent struts suggesting good vascular healing post IVL use.
How will these findings impact your approach to calcium modification?
Durability of PCI outcomes is a very important concern for interventional cardiologists. Particularly in calcified coronary artery disease which as we know is a risk factor for stent failure due to stent under-expansion. We know that adequate calcium modification improves our acute PCI results by allowing stent expansion, but the durability of the result, and avoiding re-interventions, is what is most important for patient care. Overall our findings confirm that modifying calcium using IVL results in not just good acute outcomes, but also, reassuringly, durable longer-term outcomes.
Dr. McInerney is a paid consultant for Shockwave Medical.
Coronary Important Safety Information:
In the United States: Rx only.
Indications for Use—The Shockwave Intravascular Lithotripsy (IVL) System with the Shockwave C2 Coronary IVL Catheter is indicated for lithotripsy-enabled, low-pressure balloon dilatation of severely calcified, stenotic de novo coronary arteries prior to stenting.
Contraindications—The Shockwave C2 Coronary IVL System is contraindicated for the following: This device is not intended for stent delivery. This device is not intended for use in carotid or cerebrovascular arteries.
Warnings— Use the IVL Generator in accordance with recommended settings as stated in the Operator’s Manual. The risk of a dissection or perforation is increased in severely calcified lesions undergoing percutaneous treatment, including IVL. Appropriate provisional interventions should be readily available. Balloon loss of pressure was associated with a numerical increase in dissection which was not statistically significant and was not associated with MACE. Analysis indicates calcium length is a predictor of dissection and balloon loss of pressure. IVL generates mechanical pulses which may cause atrial or ventricular capture in bradycardic patients. In patients with implantable pacemakers and defibrillators, the asynchronous capture may interact with the sensing capabilities. Monitoring of the electrocardiographic rhythm and continuous arterial pressure during IVL treatment is required. In the event of clinically significant hemodynamic effects, temporarily cease delivery of IVL therapy.
Precautions— Only to be used by physicians trained in angiography and intravascular coronary procedures. Use only the recommended balloon inflation medium. Hydrophilic coating to be wet only with normal saline or water and care must be taken with sharp objects to avoid damage to the hydrophilic coating. Appropriate anticoagulant therapy should be administered by the physician. Precaution should be taken when treating patients with previous stenting within 5mm of target lesion.
Potential adverse effects consistent with standard based cardiac interventions include– Abrupt vessel closure – Allergic reaction to contrast medium, anticoagulant and/or antithrombotic therapy-Aneurysm-Arrhythmia-Arteriovenous fistula-Bleeding complications-Cardiac tamponade or pericardial effusion-Cardiopulmonary arrest-Cerebrovascular accident (CVA)-Coronary artery/vessel occlusion, perforation, rupture or dissection-Coronary artery spasm-Death-Emboli (air, tissue, thrombus or atherosclerotic emboli)-Emergency or non-emergency coronary artery bypass surgery-Emergency or non-emergency percutaneous coronary intervention-Entry site complications-Fracture of the guide wire or failure/malfunction of any component of the device that may or may not lead to device embolism, dissection, serious injury or surgical intervention-Hematoma at the vascular access site(s)-Hemorrhage-Hypertension/Hypotension-Infection/sepsis/fever-Myocardial Infarction-Myocardial Ischemia or unstable angina-Pain-Peripheral Ischemia-Pseudoaneurysm-Renal failure/insufficiency-Restenosis of the treated coronary artery leading to revascularization-Shock/pulmonary edema-Slow flow, no reflow, or abrupt closure of coronary artery-Stroke-Thrombus-Vessel closure, abrupt-Vessel injury requiring surgical repair-Vessel dissection, perforation, rupture, or spasm. Risks identified as related to the device and its use: Allergic/immunologic reaction to the catheter material(s) or coating-Device malfunction, failure, or balloon loss of pressure leading to device embolism, dissection, serious injury or surgical intervention-Atrial or ventricular extrasystole-Atrial or ventricular capture.
Prior to use, please reference the Instructions for Use for more information on warnings, precautions and adverse events. https://shockwavemedical.com/IFU
Please contact your local Shockwave representative for specific country availability and refer to the Shockwave C2 instructions for use containing important safety information.