Shockwave Reducer
Treating the pain of refractory angina is now possible, even if your patient’s discomfort is uncontrollable by traditional medical therapies. Shockwave Reducer is an innovative technology designed to provide much-needed relief to patients suffering from the symptoms of refractory angina by creating a permanent, controlled narrowing of the coronary sinus.*
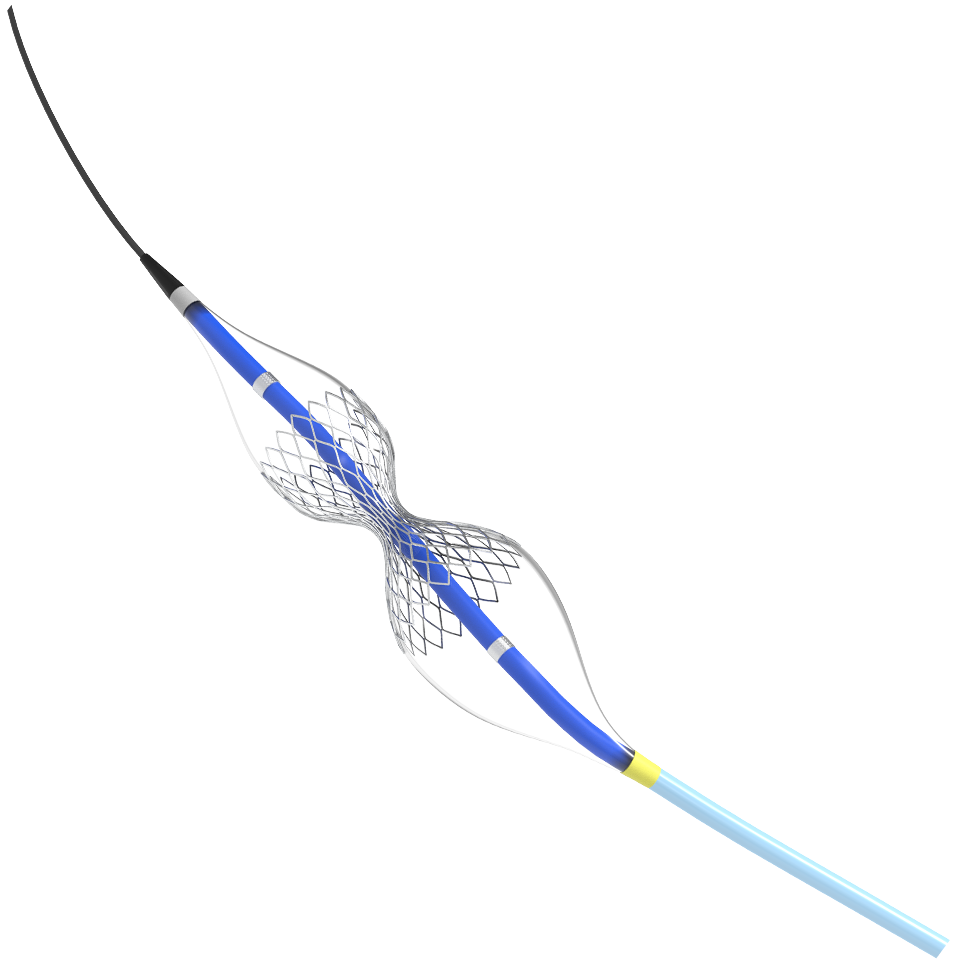

Ingeniously Designed for Angina Treatment
Millions of patients with coronary artery disease (CAD) suffer from refractory angina despite receiving optimal medical therapy, and are not candidates for revascularization. Enter Shockwave Reducer.
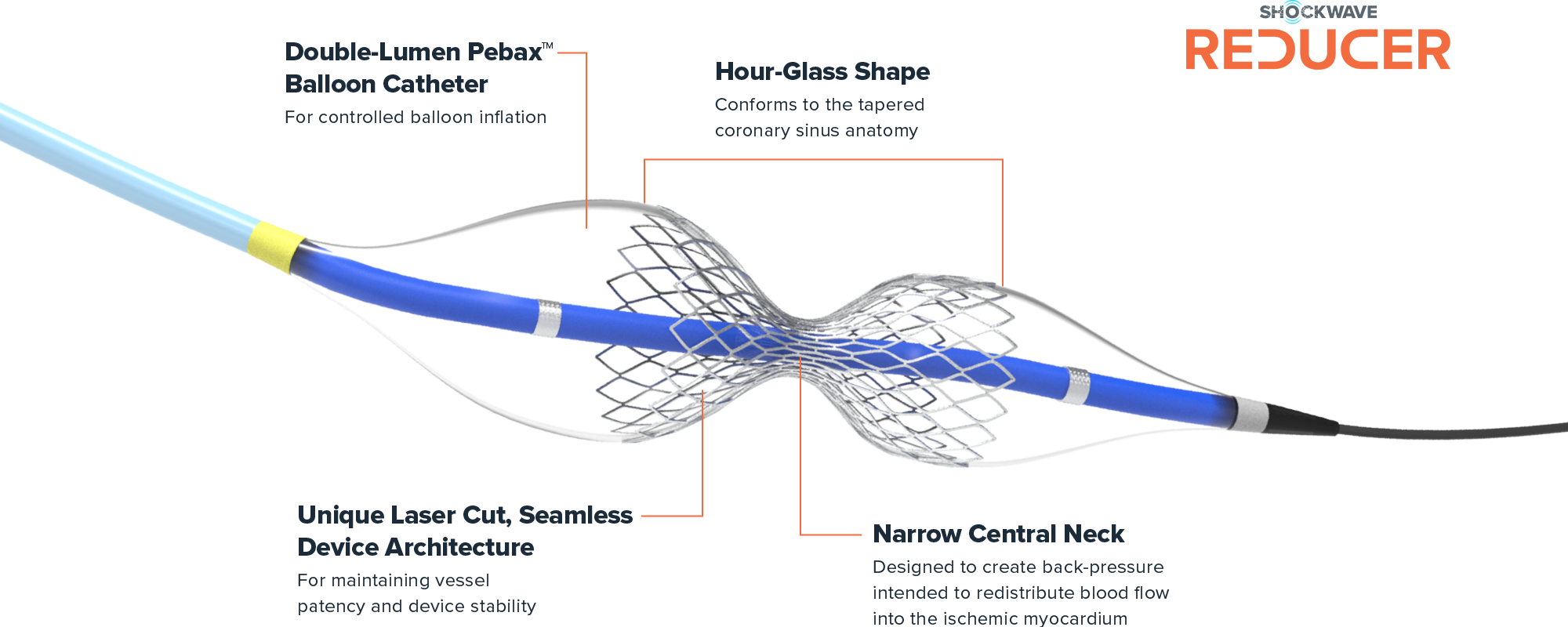
Shockwave Reducer is a small, balloon-expandable, hourglass-shaped device that establishes a narrowing in the coronary sinus. The resulting increase in back pressure reduces angina symptoms by a hypothesized redistribution of blood into the ischemic myocardium.1
Before Shockwave Reducer, there were limited options for treating refractory angina. Now, an effective, innovative solution is on hand for patients and physicians alike to improve perfusion to ischemic myocardium.
EuroPCR 2025 | Road to Relief: Effective Treatment of Refractory Angina Patients with Shockwave Reducer
At EuroPCR 2025, anchorperson James Spratt, spokesperson Felix Woitek and discussants Ranil de Silva, Mariusz Tomaniak and Tommaso Gori took the stage to present “Road to Relief: Effective Treatment of Refractory Angina Patients with Shockwave Reducer.”
In this symposium, the physicians discuss:
The physicians featured are paid consultants for Shockwave Medical.
Individual patient stories may vary.
More Shockwave Devices
1: Verheye S., et al. N Engl J Med 2015;372:519-27.
*Shockwave Reducer is commercially available in select European countries and has been implanted in over 3,500 patients. It is currently under clinical investigation in the U.S.
CAUTION: In the United States, Shockwave Reducer is an investigational device, limited by United States law to investigational use. Shockwave Reducer is subject of investigational testing and is being studied in the COSIRA-II trial in Canada. Shockwave Reducer is commercially available in certain countries outside the U.S. and Canada. Please contact your local representative for specific country availability. Prior to use, please reference the Instructions for Use for more information on warnings, precautions and adverse events: ifu.sw-reducer.com