DISRUPT BTK II
Count on it: New data confirms Shockwave Intravascular Lithotripsy’s (IVL) impact below the knee (BTK): Acute, 30-day and 6-month outcomes in the global prospective, multi-center, single-arm IVL study assessing the safety and effectiveness of the Shockwave peripheral IVL system for the treatment of calcified, stenotic BTK lesions.


Study Leadership
This section contains attributions including profile pictures, titles, descriptions, and Twitter handles.
-
Venita Chandra, MDClinical Associate Professor of Surgery, Associate Program Director – Vascular Surgery, Medical Director Stanford Advanced Wound Center
-
Ehrin Armstrong, MDMedical Director Adventist Heart and Vascular Institute
DISRUPT BTK II Study Design
Objective
Assess the continued safety and effectiveness of the Shockwave Medical peripheral IVL system for the treatment of calcified, stenotic lesions in BTK arteries. Products included Shockwave M5+ and Shockwave S4
Key Inclusion Criteria
- Rutherford classification (RC) 3-5, RC 3 capped at 20% of enrolled patients
- Moderate-severe calcification*
- Up to two BTK lesions ≤ 200 mm in length
Primary Safety Endpoint
Major adverse limb events (MALE) or post-operative death (POD) at 30-days
Primary Effectiveness Endpoint
Procedural success, defined as ≤ 50% residual stenosis for all treated target lesions without serious angiographic complications
Additional analysis to include lesions with ≤ 30% residual stenosis without angiographic complications
Independent Clinical Events Committee (CEC), Angiographic Core Laboratory, and Duplex Ultrasound Core Laboratory
* Presence of fluoroscopic evidence of calcification by PARC (Peripheral Academic Research Consortium) definition: 1) on parallel sides of the vessel and 2) extending > 50% the length of the lesion if lesion is ≥ 50 mm in length; or extending for minimum of 20 mm if lesion is < 50 mm in length.
By the Numbers
Statistics Callout
This section presents key statistical information with numbers and descriptions.
-
250Patients
-
305Lesions
-
38Global sites
-
2Year follow-up
Complex Patients with Challenging Lesions Below the Knee
Medical History
Statistics Callout
This section presents key statistical information with numbers and descriptions.
-
58.5%Patients had wounds at baseline
-
15%Hemodialysis-dependent
-
70%Diabetes mellitus
Rutherford Category
Statistics Callout
This section presents key statistical information with numbers and descriptions.
-
57%RC 5, minor tissue loss, CLTI
-
23%RC 4, ischemic rest pain, CLTI
-
20%RC 3, severe claudication*
Lesions
Statistics Callout
This section presents key statistical information with numbers and descriptions.
-
85%Moderate-severe calcification†
-
91 mmMean calcified length
-
30%Chronic total occlusions
*Enrollment of RC3 was capped at 20% | †PARC Definition
IVL’s Safety Profile Confirmed in Challenging Calcium Below the Knee
IVL was confirmed to be a safe treatment option in a challenging patient and lesion cohort.
STRONG SAFETY PROFILE
| Post-IVL | Final | |
| N | 268 lesions | 290 lesions |
| Any serious angiographic complication (total) | 1.9% (5/268) | 1.0% (3/290) |
| Dissection (Type D-F) | 0.7% (2/268) | 0.3% (1/290) |
| Total perforations | 1.5% (4/268) | 0.7% (2/290) |
| Distal embolization1 | 0% (0/268) | 0% (0/290) |
| Slow flow/no reflow2 | 0% (0/268) | 0% (0/290) |
| Abrupt closure | 0.4% (1/268) | 0.3% (1/290) |
| Thrombus | 0% (0/268) | 0% (0/290) |
Three patients with serious angiographic complications at final:
- One patient had one undiagnosed minor perforation (Grade I)
- One patient had a Grade II perforation seen pre-IVL balloon inflation, downgraded to Grade I after DES placement
- One patient experienced abrupt vessel closure despite DES placement for grade F dissection
None of the three patients had adverse events through discharge.
1: One patient had a distal thromboembolism post-IVL. Transluminal suction thrombectomy was performed and the event was successfully resolved although imaging was not provided to the core lab for assessment, therefore, data was not included in table.
2: No Reflow defined as reduced antegrade flow without evidence of residual stenosis or dissection at the treatment site.
IVL’s Effectiveness Confirmed in Challenging Calcium Below the Knee
IVL was confirmed to be effective at reducing lesion stenosis with minimal additional treatment therapy.
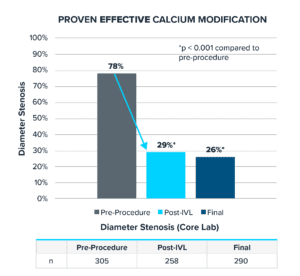
Primary Effectiveness Endpoint
- Final residual stenosis of ≤ 50% without serious angiographic complications: 97.9% (232/237, 95% CI 95.1%-99.3%)
- Additional analysis: Final residual stenosis of ≤ 30% without serious angiographic complications: 74.1% (215/290, 95% CI 68.7%-79.1%)
Procedural Information
Statistics Callout
This section presents key statistical information with numbers and descriptions.
-
65%No pre-dilation
-
65%No post-dilation
-
4.9%Total stent/tack implant
-
2.3%Provisional stent/tack implant*
*Remaining stent/tack implants were part of physicians’ standard algorithm.
Primary Safety Endpoint at 30-Days
Results for the primary safety endpoint at 30-days further confirm IVL’s safety and ongoing efficacy in challenging calcium below the knee.
| Measure | Rate | 95% CI |
| Major Adverse Limb Events (MALE) or Post-Operative Death (POD) | 0.8% (2/242) | 0.1% – 3.0% |
| All-cause death | 0.0% (2/242) | 0.0% – 1.5% |
| Above-ankle amputation of the index limb | 0.8% (2/242) | 0.1% – 3.0% |
| Major reintervention | 0.0% (2/242) | 0.0% – 1.5% |
Two patients required above-ankle amputations of the index limb:
- One patient had worsening necrotizing fasciitis of the right foot and underwent amputation nine days after index procedure
- One patient had non healing wounds in multiple areas of the foot and had an amputation nine days after index procedure
These events were reported as not related to the study device or procedure and the decision to amputate was made on clinical grounds by the treating physician.
Freedom from CD-TLR at 6-months
6-month results demonstrate durability of IVL treatment in patients with challenging calcified BTK disease.
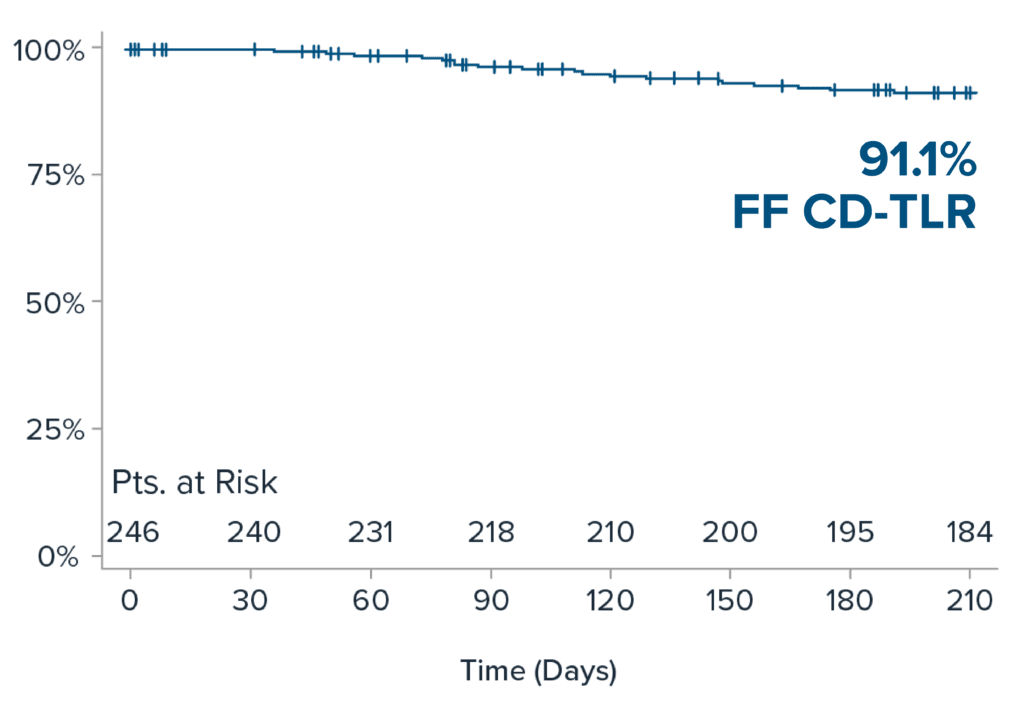
| Primary Patency* | 78.7% (111 /141) |
| Freedom from CD-TLR** | 90.7% (195/215) |
| Freedom from Restenosis via DUS | 91.7% (111/121) |
*121 patients had available and readable doppler ultrasound (DUS) while the rest did not, therefore they were excluded from the patency assessment, unless they had already failed patency due to the presence of CD-TLR. This means the 20 patients who had failed freedom from CD-TLR were added to the patency denominator totaling 141.
**In CD-TLR post-hoc analysis, patients with target limb above-the-ankle amputation were considered not eligible for CD-TLR assessment and removed from the denominator (similar to a death or patient withdrawn from the study prior to 6 months). Patients with target limb above-the-ankle amputations also censored from KM analysis of CD-TLR.
Freedom from Amputation/Death at 6-months
6-month results demonstrate durability of IVL treatment in patients with challenging calcified BTK disease.
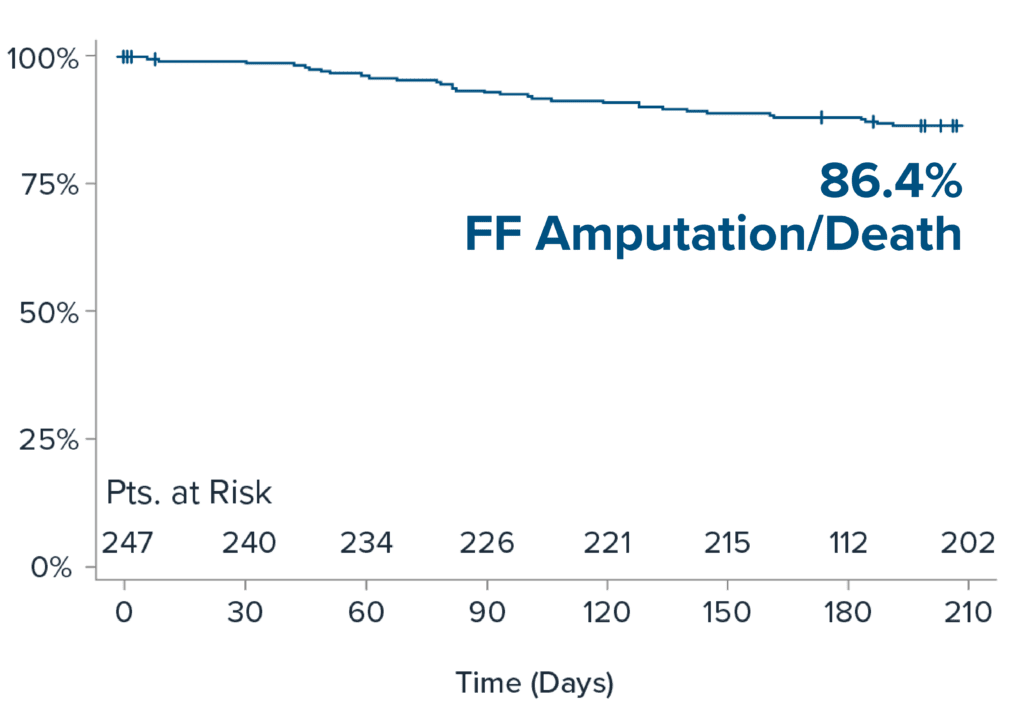
The Kaplan-Meier estimator is used to show time-to-event data. The KM estimates account for data censoring (e.g. patients are lost to follow-up). The Kaplan-Meier method allows for proper handling of censored data. It adjusts for the fact that some individuals were not observed for the entire time period, using the information available up to the point of censoring.
Rutherford Category (RC) at 6-months
In a cohort that started with 80% of patients with CLTI at baseline, at 6-months only 28.3% remain with CLTI.
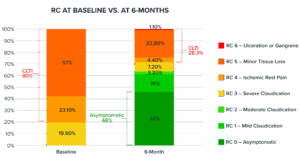
*Available data at 6-months is 180 limbs out 251 limbs.
Quality of life (QoL) at 6-months
Continued Improvement in VascuQoL-6 is clinically meaningful at 6-months.
| Baseline | 6-Months | p-value | |
| Total VascuQoL-6 Score | 12.1 ± 4.0 | 17.1 ± 5.5 | <0.001 |
Break-Down by Attribute
| VascuQoL-6 Attribute |
Average Score Baseline |
Average Score 6-Months |
| Activity | 1.9 | 2.8 |
| Tired | 2.0 | 2.7 |
| Walk | 2.3 | 3.0 |
| Concern | 1.8 | 2.8 |
| Social | 2.4 | 3.1 |
| Pain | 1.8 | 2.7 |
The Vascular Quality of Life Questionnaire is a survey used to determine health related quality of life (HRQoL) in patients with peripheral arterial disease (PAD). Each attribute is scored 1-4, a higher value indicates better health status. The sum of each individual attribute score is used to generate a total quality of life score.
Increase of 4.7 ± 5.5 in total VascuQoL-6 at 6-months
To count as a clinically meaningful improvement, patients with intermittent claudication need to improve by 2 to 3.8 points¹, while patients with CLTI need to improve by 0.48 to 0.51 points.2,3 An increase of 4.7 points in this cohort represents a clinically meaningful improvement for patients. These data add to the understanding of QoL metrics and confirm the value of IVL revascularization on patient reported outcomes.
1 Hageman et al. Eur J Vasc Endovasc Surg 2022;63:457-463.
2 Frans et al. Eur J Vasc Endovasc Surg. 2014 Feb;47(2):180-6.
3 Perlander et al. Eur J Vasc Endovasc Surg. 2023 Aug;66(2):245-251.
-

DISRUPT BTK II Acute Outcomes: Physician Perspectives with Dr. Lakshminarayan & Prof. Holden
-

DISRUPT BTK II Acute & 30-Day Outcomes with Dr. Venita Chandra and Dr. Ehrin Armstrong
More Shockwave Peripheral IVL Studies
-
Post-market, European study to assess the safety and performance of Shockwave IVL as a standalone therapy in heavily calcified infrapopliteal lesions out to 30 days.Peripheral IVL
-
The largest-ever randomized clinical study of Shockwave peripheral IVL treatment in severely calcified peripheral lesions, out to 24 months.Peripheral IVL
-
The largest prospective real-world evidence for the treatment of complex, heavily calcified peripheral arterial disease (PAD).Peripheral IVL
Chandra V, Lansky AJ, Sayfo S, et al. Thirty-Day Outcomes from the Disrupt PAD BTK II Study of the Shockwave Intravascular Lithotripsy System for Treatment of Calcified Below-the-Knee Peripheral Arterial Disease. Journal of Vascular Surgery. Published online November 12, 2024. doi:10.1016/j.jvs.2024.11.003
Holden A., Charing Cross 2025

