Shockwave IVL for Coronary Artery Disease
First-in-class safety, a uniquely sound strategy. Shockwave Coronary Intravascular Lithotripsy (IVL) is a paradigm-shifting device enabling interventional cardiologists to bridge the gap between predictably safe and consistently effective coronary calcium modification in patients suffering from calcified coronary artery disease.


The Importance of Treating Coronary Artery Disease (CAD)
Coronary artery disease is a global problem that affects approximately 315 million people every year.1 In some CAD, calcified plaque limits blood flow in the larger arteries of the heart, which increases the risk of adverse side effects.
- Over 30% of coronary lesions have severe to moderate coronary calcification2
- Coronary artery calcification increases procedural complications,3, 4, 5, 6, 7 impedes stent delivery8 and prevents stent expansion2, 9
Shockwave Coronary IVL disrupts coronary calcium through its unique mechanism of action (MOA), modifying calcium in a safe, effective and intuitive manner to help restore vessel compliance prior to stent deployment.
See How Shockwave IVL Performs Across Calcium Morphologies
-

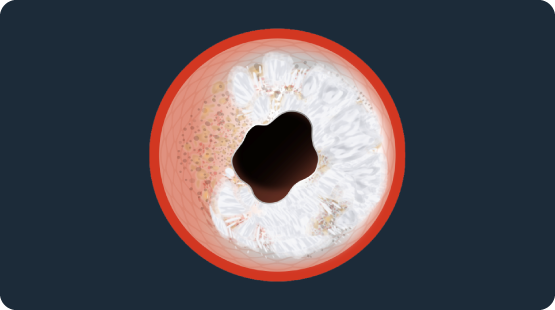
Shockwave Coronary IVL & Concentric Calcium
-

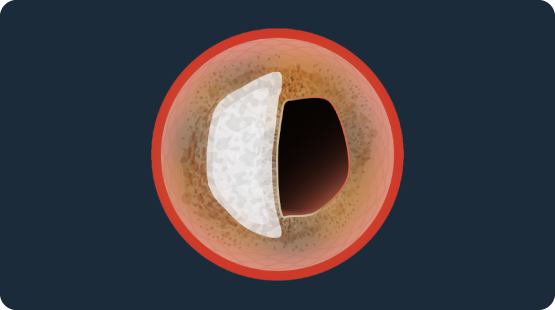
Shockwave Coronary IVL & Eccentric Calcium
-

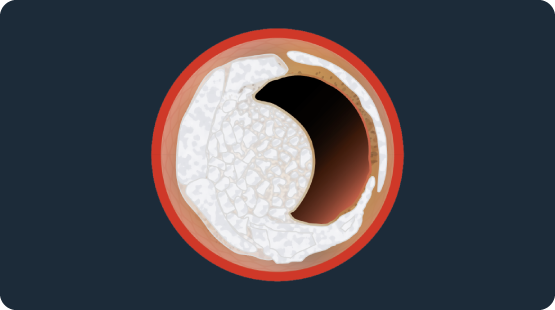
Shockwave Coronary IVL & Nodular Calcium
First-in-class Safety, A Uniquely Sound Strategy
Shockwave Medical is the first-ever manufacturer of Coronary IVL products — the most studied and frequently used calcium modification strategy10 in our field. Our commitment to clinical research, continuous innovation and meaningful physician collaboration has set a new standard of care that is shaping the future of treating calcified coronary artery disease.
Statistics Callout
This section presents key statistical information with numbers and descriptions.
-
305Peer-reviewed journal publications
-
7,949Published patient outcomes
-
600,000+Patients treated
| Single-arm Studies | Real-world Registries | |||||
| FINAL ANGIOGRAPHIC COMPLICATIONS | DISRUPT CAD I11 | DISRUPT CAD II12 | DISRUPT CAD III13 | DISRUPT CAD IV14 | Shock India15 | France Lili16 |
| Patients | 60 | 120 | 384 | 64 | 1,053 | 500 |
| Severe dissection categorization (D-F) | 0% | 0% | 0.3% | 0% | 0% | 0.2% |
| Perforation | 0% | 0% | 0.3% | 0% | 0.2% | 0.6% |
| Abrupt closure | 0% | 0% | 0.3% | 0% | 0.1% | 0.2% |
| Slow flow/no reflow | 0% | 0% | 0% | 0% | 0.1% | NR |
NR = not reported
-
Head-to-Head ROLLING-STONE Registry Showcases Safety of IVL over Atherectomy
The ROLLING-STONE Registry is the largest, prospective, multi-center registry with a head-to-head comparison after propensity score matching (PSM) of Shockwave IVL versus atherectomy (AT) in a real-world, all-comers population. The 1,005 patient registry compared procedural success, intraprocedural complications and 30-day and 1-year MACE rates after PSM of IVL versus rotational atherectomy (RA) and orbital atherectomy (OA).
-
ROLLERCOASTR-EPIC22 Trial: The Importance of Device Selection
The ROLLERCOASTR-EPIC22 Trial* is a prospective, randomized controlled trial comparing the safety and efficacy of Shockwave IVL, rotational atherectomy (RA) and laser atherectomy (ECLA) within moderate and severely calcified lesions. The trial demonstrates a lower procedural complication rate with Shockwave IVL with similar procedural success, minimum stent area (MSA) and stent expansion.
Not the Same Old Drill: The Leading Solution for Treating Calcified CAD
Use of Calcium Modification During Percutaneous Coronary Intervention After Introduction of Coronary Intravascular Lithotripsy10
Coronary IVL is now the most frequently used coronary calcium modification strategy for treatment of calcified coronary lesions. An analysis of the National Cardiovascular Data Registry CathPCI Registry demonstrates a steady, significant increase in usage since the device’s U.S. commercial launch in 2021, showcasing a previous unmet need for a safe, effective and intuitive calcified lesion preparation strategy.
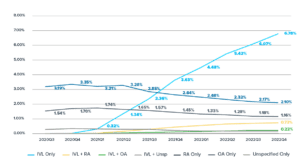
*The Rollercoastr-EPIC22 Trial is not sponsored by Shockwave Medical.
1: Stark, B., Johnson, C., & Gregory Andrew Roth. (2024). Global Prevalence of Coronary Artery Disease: An Update from The Global Burden of Disease Study. Journal of the American College of Cardiology, 83(13), 2320-2320.
2: Généreux P, et al. JACC 2014; 63(18);1845-54.
3: Madhavan MV, Tarigopula M, Mintz GS, Maehara A, Stone GW, Généreux P. Coronary artery calcification: pathogenesis and prognostic implications. J Am Coll Cardiol 2014;63:1703.
4: Chambers JW, Feldman RL, Himmelstein SI, et al. Pivotal trial to evaluate the safety and efficacy of the Orbital Atherectomy System In Treating De Novo, Severely Calcified Coronary Lesions (ORBIT II). J Am Coll Cardiol Intv 2014;7:510-8.
5: Genereux P, Lee AC, Kim CY, et al. Orbital atherectomy for treating de novo severely calcified coronary narrowing (1-year results from the pivotal ORBIT II Trial). Am J Cardiol 2015;115: 1685-90.
6: Yamamoto MH, Maehara A, Karimi Galougahi K, et al. Mechanisms of orbital versus rotational atherectomy plaque modification in severely calcified lesions assessed by optical cohérence tomography. J Am Coll Cardiol Intv 2017;10: 2584-6.
7: Kini AS, Vengrenyuk Y, Pena J, et al. Optical coherence tomography assessment of the mechanistic effects of rotational and orbital atherectomy in severely calcified coronary lesions. Catheter Cardiovasc Interv 2015;86:1024-32.
8: Ferrer Gracia MC, et al. Failure in the implantation of drug eluting stents. Frequency and related factors. Med Intensiva. 2007.
9: Mintz, G; I. J Am Coll Cardiol Imaging 2015;8(4): 461-71.
10: Neel Butala et al. “Use of Calcium Modification During Percutaneous Coronary Intervention After Introduction of Coronary Intravascular Lithotripsy” Journal of the Society for Cardiovascular Angiography & Interventions. 2024; DOI: 10.1016/j.jscai.2023.101254.
11: Brinton, T.J., et al. Circulation. 2019;139:834-36.
12: Ali, Z.A., et al. Circ Cardiovasc Interv. 2019;12:e008434.
13: Hill, J.M., et al. J Am Coll Cardiol. 2020;76:2635-46.
14: Saito, S., et al. Circ J. 2021;85:826-33.
15: Ashok , S. (2023, October). A Prospective, Observational, Single Arm, Multicenter Registry to evaluate clinical outcomes of the Shockwave Coronary Intravascular Lithotripsy (IVL) followed by stent implantation in Calcified Coronary Arteries in Real World Indian Patient Population (Shock India Registry). Transcatheter Cardiovascular Therapeutics (TCT) 2023. A research grant was provided by Shockwave Medical for the Shock India Registry.
16: Honton, B., et al. Archives of Cardiovascular Disease. 2024; 117 (2024) S3-S29. A research grant was provided by Shockwave Medical for the France Lili Registry.
Definitions of clinical outcomes and/or endpoints differ across studies.