DISRUPT CAD I CE Mark Study
A single-arm, pre-market study demonstrating the safety and performance of Shockwave Intravascular Lithotripsy (IVL) in heavily calcified, coronary lesions prior to stenting and followed to 6 months; also included an OCT sub-study demonstrating Shockwave IVL’s mechanism of action.


Study Leadership
This section contains attributions including profile pictures, titles, descriptions, and Twitter handles.
-
Jean Fajadet, MDClinical Pasteur, Toulouse, France
-
Prof. Carlo Di MarioAOU Careggi SOD Interventistica, Firenze, Italy
Statistics Callout
This section presents key statistical information with numbers and descriptions.
-
60Patients
-
7European sites
-
100%Severe calcified lesions
-
6Month follow-up
Study Design and Patient Characteristics
Objective
A single-arm, pre-market study demonstrating the safety and performance of Shockwave IVL in heavily calcified, coronary lesions prior to stenting and followed to 6 months; also included an OCT sub-study demonstrating Shockwave IVL’s mechanism of action.
Primary Safety Endpoint
30-day major adverse cardiovascular event (MACE)
Secondary Performance Endpoint
<50% residual stenosis post-percutaneous coronary intervention (PCI) with no evidence of in-hospital MACE
Patient Characteristics Summary
- Stable angina, unstable angina or silent ischemia
- Moderate and severely calcified, de novo coronary lesions right ventricular systolic dysfunction (RVD) 2.5 – 4.0 mm, stenosis ≥50%, lesion length ≤32 mm
Countries Included in Study
-
 Australia
Australia
-
 France
France
-
 Netherlands
Netherlands
-
 Sweden
Sweden
-
 United Kingdom
United Kingdom
Strong Safety and Low Complications
| Safety | Results | Events |
| 30-day MACE* cardiac death, myocardial infarction (MI) or tricuspid valve replacement (TVR) | 5% | Cardiac death N = 0 QWMI N = 0 **NQWMI N = 3 TVR N = 0 |
| 6-month MACE* cardiac death, myocardial infarction (MI) or tricuspid valve replacement (TVR) | 8.3% | Cardiac death N = 2 QWMI N = 0 **NQWMI N = 3 TVR N = 0 |
Core lab adjudicated
*CEC adjudicated
**NQMI defined as 3x upper limit CK-MB
| Complications | Procedural | Final |
| Dissection (D / E / F) | 3.3% / 0% / 0% | 0% / 0% / 0% |
| Perforation | 0.0% | 0.0% |
| Abrupt closure | 0.0% | 0.0% |
| Slow flow | 0.0% | 0.0% |
| No reflow | 0.0% | 0.0% |
Brinton et al 2019 in Circ Int.
DISRUPT CAD I OCT Sub-Study Confirms Mechanism of Action (MOA) of Shockwave Intravascular Lithotripsy
Statistics Callout
This section presents key statistical information with numbers and descriptions.
-
31Patients
-
3OCT runs: Baseline, post-IVL & post-stent
-
55%Fracture post-stent
-
2.1 mm2Acute area gain post-IVL
-
5.9 mm2Minimal stent area
Read the full DISRUPT CAD I OCT sub-analysis on the JACC: Cardiovascular Imaging website:
OCT Sub-Analysis Key Findings
- Transmural fractures: Shockwave IVL shown to fracture both intimal and medial calcium under OCT
- More fractures: The more severe the calcium, the greater the incidence of fracture
- Improved compliance: Improved vessel compliance enables similar stent expansion across all lesions, despite calcium severity
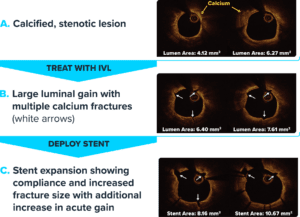
Frames are co-registered to ensure cross-sections are in the same location.
More DISRUPT CAD Studies
-
European post-market study confirming DISRUPT CAD I results and showing strong safety and procedural success with Shockwave coronary IVL.Coronary IVL
-
Largest and most rigorous Shockwave study showcasing the safety, effectiveness and ease of use of Shockwave coronary IVL .Coronary IVL
-
Shockwave coronary study demonstrating the safety and effectiveness of Shockwave IVL within a Japanese patient population.Coronary IVL