DISRUPT CAD II
European post-market study confirming DISRUPT CAD I results and showing strong safety and procedural success with Shockwave coronary Intravascular Lithotripsy (IVL).


Study Leadership
This section contains attributions including profile pictures, titles, descriptions, and Twitter handles.
-
Prof. Jean FajadetCo-Principal Investigator Clinique Pasteur, Toulouse, France
-
Prof. Carlo di MarioCo-Principal Investigator AOU Careggi SOD Interventistica Firenze, Italy
Statistics Callout
This section presents key statistical information with numbers and descriptions.
-
120Patients
-
15Hospitals
-
94%Severe Ca++
-
100%Successful delivery of Shockwave IVL
-
100%Successful delivery of stents
-
0%Severe dissections
-
0%Perforations
-
0%Slow/no reflow
Study Design and Patient Characteristics
Objective
A post-market study to assess the safety and performance of the coronary Shockwave IVL system with more patients in more centers
Primary Safety Endpoint
In-hospital major adverse cardiovascular event (MACE) (cardiac death, myocardial infarction or target vessel revascularization)
Secondary Performance Endpoint
Clinical and angiographic success
Patient Characteristics Summary
- 72 years old
- 32% diabetes mellitus (DM)
- 3.0 mm reference vessel diameter (RVD)
- 60% diameter stenosis
- 26 mm Ca++ length
- 72% concentric/28% eccentric lesions
- 30% side branch involvement
Countries Included in Study
-
 Belgium
Belgium
-
 Denmark
Denmark
-
 France
France
-
 Germany
Germany
-
 Italy
Italy
-
 Netherlands
Netherlands
-
 Spain
Spain
-
 Sweden
Sweden
-
 United Kingdom
United Kingdom
Strong Performance and Safety with Low Complications
Accordion Section
| Performance Outcomes | Results |
| Clinical success | 94.2% (113) |
| Angiographic success | 100% (120) |
| Stent delivery | 100% (120) |
Clinical success: ability of Shockwave IVL to produce a residual diameter stenosis <50% after stenting with no evidence of in-hospital MACE.
| Final In-Stent Angiographic Outcomes (Core Lab) | Results |
| Residual diameter stenosis, % | 7.8% ± 7.1 |
| Acute gain, mm | 1.67 ± 0.49 |
| Residual diameter stenosis <50% | 100% (120) |
| Residual diameter stenosis <30% | 100% (120) |
| Safety Outcomes | Results |
| Final angiographic complications | 0.0% (0/120) |
| Dissections, type D-F | 0.0% (0/120) |
| Perforations | 0.0% (0/120) |
| Abrupt closure | 0.0% (0/120) |
| Slow flow | 0.0% (0/120) |
| No reflow | 0.0% (0/120) |
| Major adverse cardiac events in-hospital | 5.8% (7/120) |
| Cardiac death | 0.0% (0/120) |
| Non-Q-wave myocardial infarction | 5.8% (7/120) |
| Q-wave myocardial infarction | 0.0% (0/120) |
| Target vessel revascularization | 0.0% (0/120) |
| Major adverse cardiac events through 30 days* | 7.6% (9/119) |
| Cardiac death | 0.8% (1/119) |
| Non-Q-wave myocardial infarction | 5.9% (7/119) |
| Q-wave myocardial infarction | 0.8% (1/119) |
| Target vessel revascularization | 0.8% (1/119) |
| Stent thrombosis (definite or possible) | 1.7% (2/119) |
*10 MACE in 9 subjects
| DISRUPT CAD II OCT Sub-Study | Results |
| Calcium fracture, % | 78.7% (37/47) |
| Calcium fracture per lesion | 3.4% ± 2.6 |
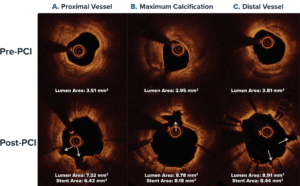
OCT Case Example: Proximal Left Anterior Descending Artery (LAD) with Side Branch Involvement
- Severe Ca++: >270° and >1 mm thick
- Ca++ fractures: Multiple locations (white arrows)
- Final result: Full stent expansion large acute gain
More DISRUPT CAD Studies
-
Single-arm, pre-market study demonstrating the safety and performance of Shockwave IVL in heavily calcified coronary lesions.Coronary IVL
-
Largest and most rigorous Shockwave study showcasing the safety, effectiveness and ease of use of Shockwave coronary IVL .Coronary IVL
-
Shockwave coronary study demonstrating the safety and effectiveness of Shockwave IVL within a Japanese patient population.Coronary IVL