DISRUPT BTK II Acute & 30-Day Outcomes with Dr. Venita Chandra and Dr. Ehrin Armstrong
In this VIVA 2024 interview, Dr. Nick West, Chief Medical Officer of Shockwave Medical, moderates a discussion on the DISRUPT BTK II acute and 30-day outcomes with the two co-principal investigators of the study, Dr. Armstrong, Interventional Cardiologist and Medical Director at Adventist Heart & Vascular Institute, and Dr. Venita Chandra, Vascular Surgeon and Clinical Professor at Stanford Health Care.
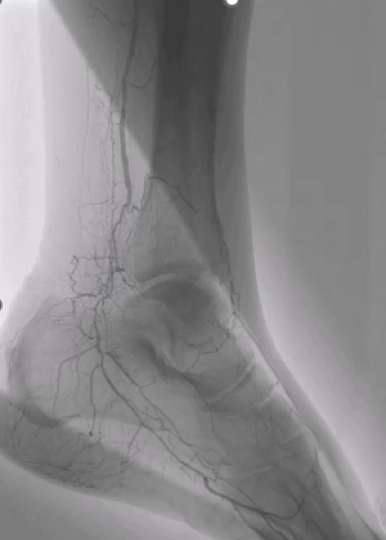
Watch now to learn more about the study’s significance, particularly in addressing an understudied area—below-the-knee (BTK) disease in patients with critical limb-threatening ischemia (CLTI). The study includes high-risk patients with heavy calcification who are often excluded from similar trials.
The 30-day results show promising outcomes, with low Major Adverse Limb Events (MALEs) and no mortality. A majority of patients experienced a substantial reduction in stenosis, with more than 90% achieving less than 50% residual stenosis and approximately 75% reaching less than 30% residual stenosis—all with a low stent rate (less than 5%) and low angiographic complications (1.0% at Final). Additionally, early wound healing signals were observed, and a mean improvement in Vascular Quality of Life scores within 30 days. The investigators are particularly excited about the upcoming six-month and one-year data, which will provide more insight into long-term wound healing and vessel patency.
Chandra V, Lansky AJ, Sayfo S, et al. Thirty-Day Outcomes from the Disrupt PAD BTK II Study of the Shockwave Intravascular Lithotripsy System for Treatment of Calcified Below-the-Knee Peripheral Arterial Disease. Journal of Vascular Surgery. Published online November 12, 2024. doi:10.1016/j.jvs.2024.11.003.
Dr. Armstrong and Dr. Venita Chandra are paid consultants of Shockwave Medical.
Shockwave IVL: In the U.S.: Rx only. Prior to use, please reference the Important Safety Information for more information on indications, contraindications, warnings, precautions and adverse events.