DISRUPT BTK II Acute Outcomes: Physician Perspectives with Prof. Holden & Dr. Lakshminarayan
Join Dr. Nick West, Chief Medical Officer of Shockwave Medical, Professor Andrew Holder, Auckland City Hospital, New Zealand, and Dr. Raghuram Lakshminarayan, Hull Royal Infirmary, UK, as they discuss the DISRUPT BTK II acute outcomes data at CIRSE 2024.
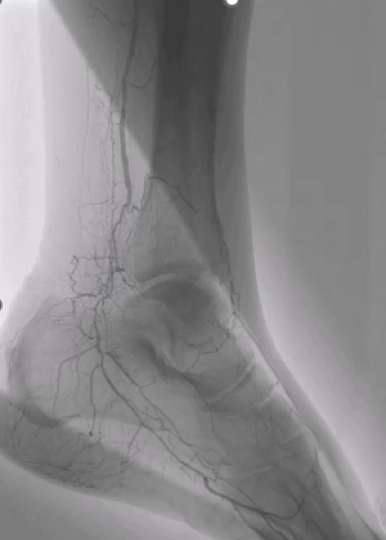
This discussion focuses on the procedural outcomes of the DISRUPT BTK II trial which studied the treatment of Intravascular Lithotripsy (IVL) for calcified below-the-knee (BTK) arterial disease. The study, which is the largest of its kind with 250 patients, focuses on treating severely calcified lesions in a complex patient population, including a high percentage of patients with diabetes and those with renal impairment. The findings highlight IVL’s strong safety profile and efficacy, with a low rate of angiographic complications and a high success rate in achieving significant luminal gain without the need for stenting in most cases.
The experts conclude that these results validate IVL’s role in BTK interventions and may encourage its broader adoption, particularly with the introduction of new IVL catheter technologies for endovascular treatments.
Chandra V, Lansky AJ, Sayfo S, et al. Thirty-Day Outcomes from the Disrupt PAD BTK II Study of the Shockwave Intravascular Lithotripsy System for Treatment of Calcified Below-the-Knee Peripheral Arterial Disease. Journal of Vascular Surgery. Published online November 12, 2024. doi:10.1016/j.jvs.2024.11.003.
Professor Andrew Holder and Dr. Raghuram Lakshminarayan are paid consultants of Shockwave Medical.
Shockwave IVL: In the U.S.: Rx only. Prior to use, please reference the Important Safety Information for more information on indications, contraindications, warnings, precautions and adverse events.