Shockwave Reducer Overview & MOA
Redistribute flow. Relieve pain. Restore hope. Shockwave Reducer’s mechanism of action (MOA) helps improve the symptoms of refractory angina by creating a permanent, controlled narrowing of the coronary sinus.*
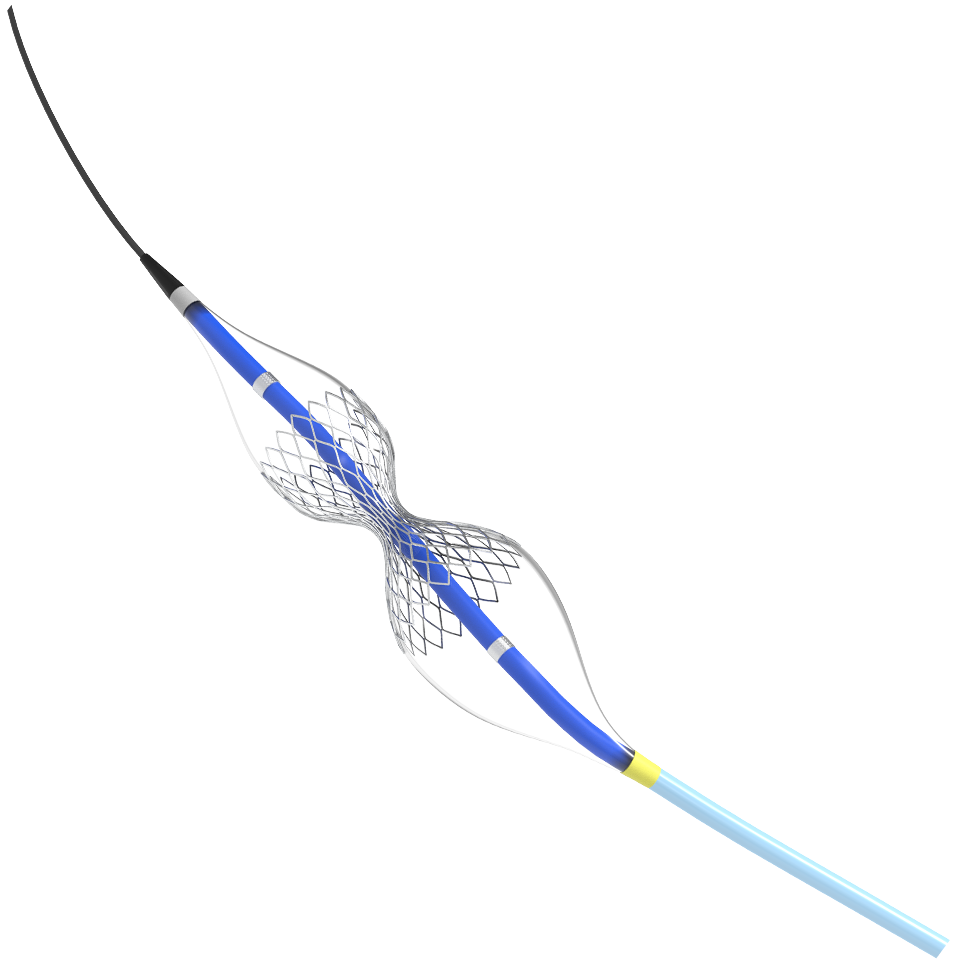

Meet Shockwave Reducer: A Small Device Offering Big Relief
Shockwave Reducer is a small, balloon-expandable, hourglass-shaped device that establishes a narrowing in the coronary sinus. The resulting increase in back pressure reduces angina symptoms by a hypothesized redistribution of blood into the ischemic myocardium.1
How Shockwave Reducer helps redistribute flow from epicardium to endocardium:
- Shockwave Reducer device is implanted into the coronary sinus
- Resistance to venous drainage is increased
- As with any blocked stream, pressure increases backwards to the venous system
- This back pressure effect extends upstream along the venous drainage, increasing pressure and causing microvasculature to expand
- The outcome is redistribution of flow, enabling renewed perfusion in the ischemic areas of the subendocardium
ORBITA-COSMIC: Providing Insights into Shockwave Reducer’s MOA
ORBITA-COSMIC was a randomized, placebo-controlled study demonstrating how Shockwave Reducer reduces angina frequency and improves heart disease related quality of life while improving subendocardial perfusion.2
51 patients were randomized at six centres in the United Kingdom. Shockwave Reducer decreased the number of daily angina episodes compared to placebo at six months.
- Imaging endpoint showed improvement in subendocardial perfusion, supporting the theorized mechanism of action of Shockwave Reducer
- In ischaemic segments, the endocardial to epicardial ratio of stress myocardial blood flow improved in the Shockwave Reducer group with a 98.2% probability of benefit
Shockwave Reducer Resources
*Shockwave Reducer is commercially available in select European countries and has been implanted in over 3,500 patients. It is currently under clinical investigation in the U.S.
**Prof. Spratt is a paid consultant of Shockwave Medical.
CAUTION: In the United States, Shockwave Reducer is an investigational device, limited by United States law to investigational use.
Shockwave Reducer is subject of investigational testing and is being studied in the COSIRA-II trial in Canada.
Shockwave Reducer is commercially available in certain countries outside the U.S. and Canada. Please contact your local representative for specific country availability.
Prior to use, please reference the Instructions for Use for more information on warnings, precautions and adverse events: ifu.sw-reducer.com
1: Verheye S., et al. N Engl J Med 2015;372:519-27.
2: Foley et al. The Lancet. 2024 Apr 8; https://doi.org/10.1016/S0140-6736(24)00256-3.
3: https://www.tctmd.com/NEWS/CORONARY-SINUS-REDUCER-EASES-SYMPTOMS-REFRACTORY-ANGINA-ORBITA-COSMIC.

