Shockwave M5+
A versatile calcium-cracking tool: Shockwave M5+ takes on calcified peripheral artery disease (PAD) using a low-risk, proven mechanism of action (MOA). The do-it-all design makes Shockwave M5+ a sound solution for a variety of patients with calcified PAD.



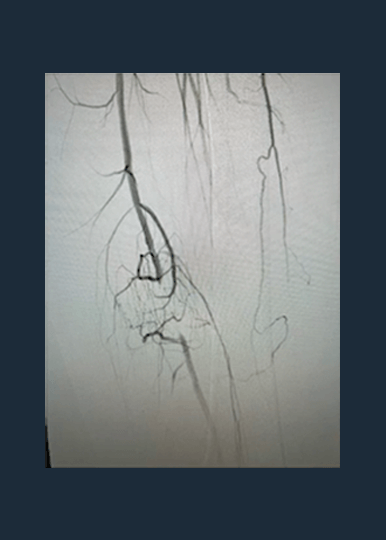
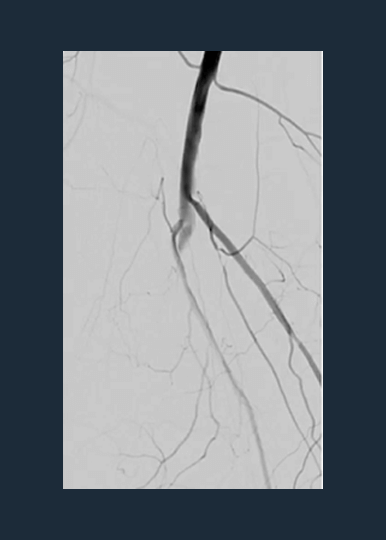
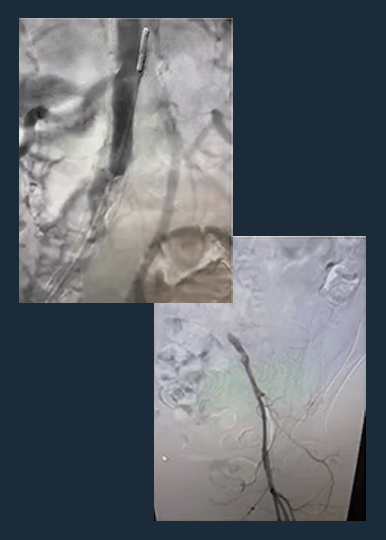
Shockwave M5+ Cracks the Complexities of Peripheral Calcium
Shockwave M5+ is a versatile catheter, effective for above-the-knee (ATK) endovascular treatments.
- Balloon length: 60 mm
- Catheter working length: 135 cm
- Balloon diameters: 7.0 and 8.0 mm
- Sheath compatibility: 7.0 – large lumen 6 Fr*, 8.0 – 7 Fr
- Total pulses: 300
Shockwave M5+ Specifications
| Catalog Number | Diameter (mm) | Balloon Length (mm) | Sheath Compatibility | Working Length (cm) | Balloon Crossing Profile Range (in) | Pulses/Cycle | Pulses (max) |
| M5PIVL7060 | 7.0 | 60 | 6 Fr* | 135 | 0.068 | 30 | 300 |
| M5PIVL8060 | 8.0 | 60 | 7 Fr | 135 | 0.074 | 30 | 300 |
*6 Fr compatible with Terumo Pinnacle® Destination® Guiding Sheath and Cook Flexor® Ansel Guiding Sheath. All trademarks, logos and brand names are the property of their respective owners or holders.
Disrupt PAD III Observational Study
The largest prospective real-world evidence for the treatment of complex, heavily calcified PAD
Statistics Callout
This section presents key statistical information with numbers and descriptions.
-
1,373Patients
-
1,531Lesions
-
30Sites
-
3Countries