Coronary Sinus Reduction for Refractory Angina
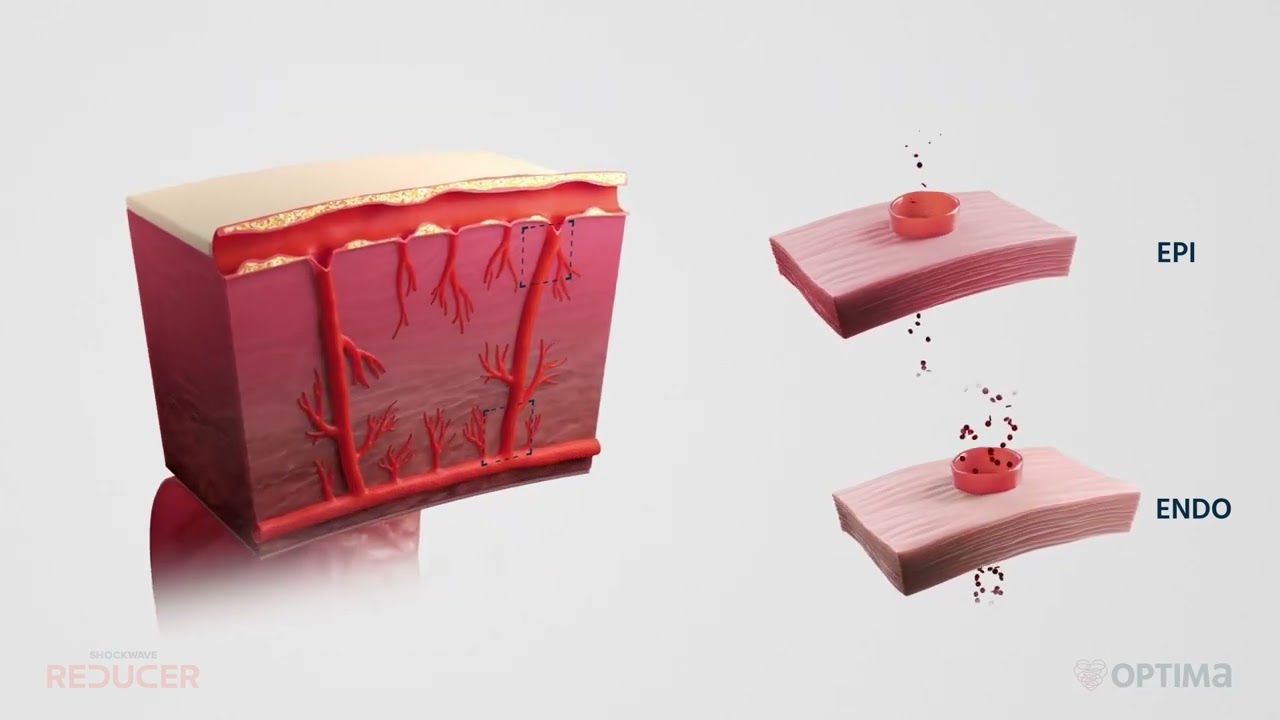
Reducing pain. Restoring hope. Millions of patients with coronary artery disease (CAD) suffer from refractory angina despite receiving optimal medical therapy. Shockwave Reducer is designed to improve the symptoms of refractory angina through a permanent, controlled narrowing of the coronary sinus.*
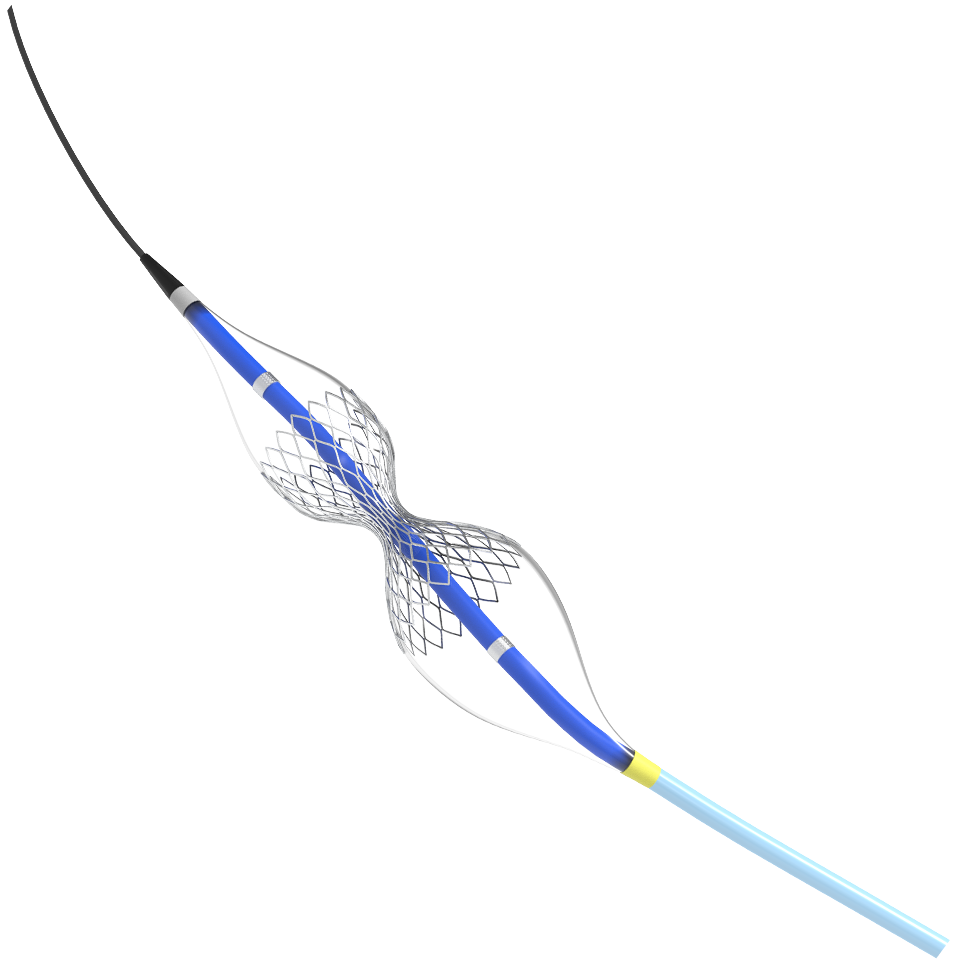

Creating Better Outcomes: Percutaneous Treatment Option for Refractory Angina
Usually a symptom of myocardial ischemia (a lack of blood flow to the heart muscle), angina may feel like pressure or squeezing in the chest and/or pain in the shoulders, arms, neck, jaw or back. Many patients experiencing angina suffer symptoms that are severe, long-lasting and uncontrollable by traditional medical therapies. This severely debilitating condition is known as refractory angina.
- Angina pain is often a symptom of CAD, when plaque buildup occurs in the arteries supplying the oxygen-rich blood to the heart, forcing the heart to work harder.
- Many patients can get relief from their angina through revascularization from a coronary intervention or surgery. However, 25–40% continue to suffer from angina even after successful revascularization.1, 2
- Angina with nonobstructive coronary arteries (ANOCA) is increasingly recognized and may affect nearly one-third of patients undergoing invasive coronary angiography for suspected CAD.3, 4 These patients do not have plaque buildup as a cause for their angina, and currently have limited options.
Mounting Clinical Evidence: Study Results
Accordion Section
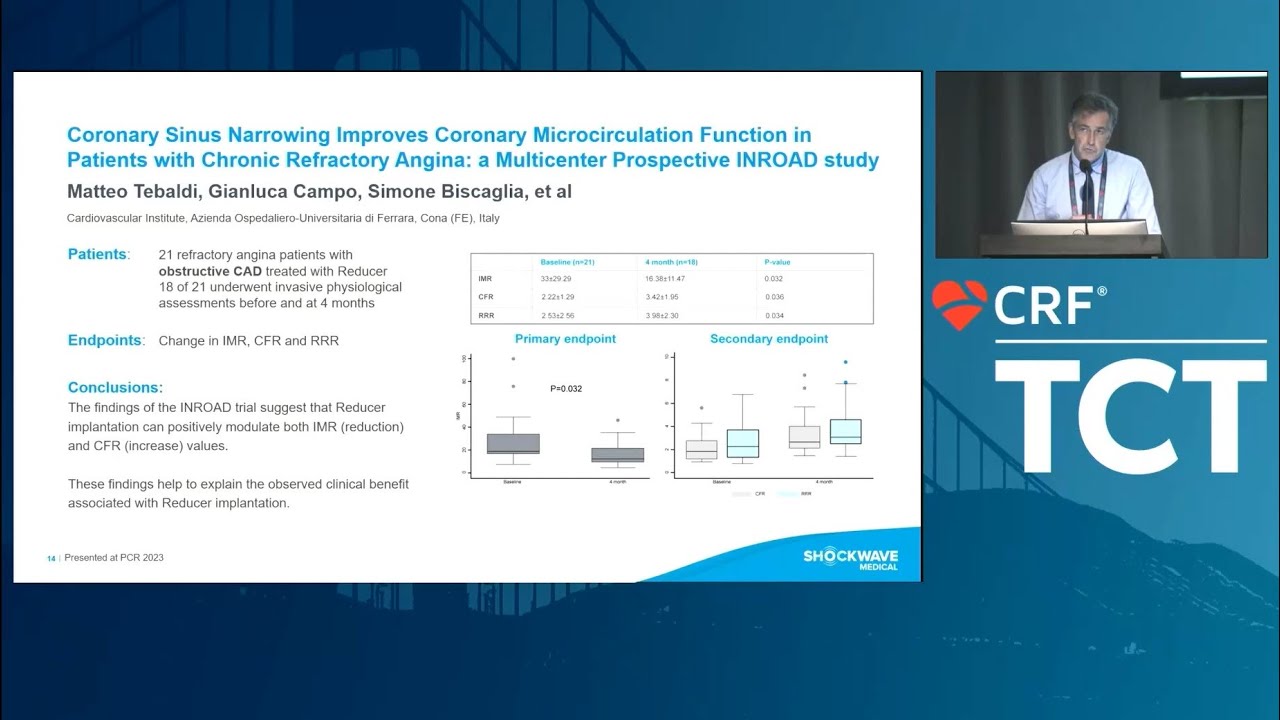
REDUCER-I 12-month results demonstrated the safety and efficacy of the Reducer in improving angina severity and quality of life in patients suffering from refractory angina.5 Additionally, the outcomes showed a reduction in emergency department visits after Reducer implantation as well as clinically significant increase in the 6 Minute Walk Test distance. The results of the 400 patients enrolled at 25 centres and followed up to three years further augmented the findings from the initial COSIRA trial.6
- 71% of patients improved one or more CCS angina classes at 12 months†
- 26% of patients improved two or more CSS angina classes at 12 months†
REDUCER-I sub-analysis of patients with non-obstructive CAD* receiving a Reducer device showed a statistically significant improvement in Quality of Life (QoL) and an improvement in CCS class at 12 months.
Non-obstructive CAD defined as less than 70% stenosis by visual estimate in all major epicardial coronary arteries.
- Both obstructive CAD (p<0.0001) and non-obstructive CAD (p<0.0002) cohorts had significant improvements in Mean SAQ Summary Score at 12 months
- Both obstructive CAD and non-obstructive CAD cohorts had improved CCS class at 12 months with no significant differences (p=0.1178) between the groups
REDUCER I sub-analysis of patients with Diabetes Mellitus (DM) showed similar improvement in angina reduction (change in Canadian Cardiovascular Society Class (CCS)), similar Quality of Life (QoL) improvements and no difference in MACE-free survival at 12 months than the patients without history of DM.
- Patients with DM (71% improvement by ≥1 CCS class) and without DM (68% improvement by ≥1 CCS class) showed similar improvement in CCS class (p=0.66)
- Patients with and without DM showed similar QoL improvements in the mean Seattle Angina Questionnaire (SAQ) summary score at 12 months (p=0.27)
REDUCER I sub-analysis of female and male patients showed similar, significant improvement in angina reduction (change in Canadian Cardiovascular Society Class (CCS)), significant vs baseline and no difference in MACE-free survival at 12 months among both cohorts.
- Both female and male cohorts had improved CCS class at 12, 24 and 36 months with no significant differences between the groups, except at 6-months, favoring females (p=0.03).
- Both female and male cohorts had significant SAQ improvements compared to baseline (p≤0.001).
Download sex specific sub-analysis data
†Paired analysis for those with both baseline AND 12 month CSS class assessment.
*CAD = coronary artery disease
Results of the independent ORBITA-COSMIC trial show Shockwave Reducer lessens angina frequency and improves heart disease related quality of life while improving subendocardial perfusion.7 51 patients were randomized at six centres in the UK. Shockwave Reducer decreased the number of daily angina episodes compared to placebo at six months.
- The results showed evidence of benefit on angina frequency reported using the smartphone ORBITA-app in the coronary sinus reducer (CSR) group compared to the placebo group
- Heart disease-related quality of life measured by MacNew Heart Disease Health-Related Quality of Life scores improved in the CSR group compared with the placebo group
- While the primary imaging endpoint showed no improvement in transmural myocardial perfusion, it did show improvement in subendocardial perfusion, supporting the theorized mechanism of action of the Shockwave Reducer
Beginning in 2010, the Reducer team and clinicians worldwide began studying the safety and effectiveness of the Shockwave Reducer device. 104 patients with Canadian Cardiovascular Society (CCS) class III or IV angina and myocardial ischemia who were not candidates for revascularization were enrolled. One randomized group received the device (treatment group) while another received a sham procedure (control group). COSIRA** demonstrated patients receiving the Shockwave Reducer device achieved a statistically significant improvement in angina symptoms and quality of life compared to patients in a sham control group.6
- 71% of patients improved 1 or more CCS angina classes at 6 months
- 35% of patients improved 2 or more CCS angina classes at 6 months
- Quality of life improved 17.6 points for patients who received the implant, vs. 7.6 points for patients in the control group
**COSIRA = COronary SInus Reducer for treatment of Refractory Angina
Building on Clinical Data: COSIRA II Study
Currently enrolling: COSIRA** II is the next-phase clinical trial designed to gather further evidence of the safety and effectiveness of Shockwave Reducer. The study is a U.S.-based, multicenter, randomized, double-blind, sham-controlled trial. ClinicalTrials.gov Identifier: NCT05102019.
**COSIRA = COronary SInus Reducer for treatment of Refractory Angina
-

COSIRA II Symposium: The Coronary Sinus Reducer
-

COSIRA II Symposium: Angina: The Scope of the Challenge
-

COSIRA II Symposium: The Future of Clinical Data for the Reducer
The physicians featured are paid consultants for Shockwave Medical.
*Shockwave Reducer is commercially available in select European countries and has been implanted in over 3,500 patients. It is currently under clinical investigation in the U.S.
Caution: In the United States, Shockwave Reducer is an investigational device, limited by United States law to investigational use. The Reducer is subject of Investigational testing and is being studied in the COSIRA-II trial in Canada.
Shockwave Reducer is commercially available in certain countries outside the U.S. and Canada. Please contact your local representative for specific country availability. Prior to use, please reference the Instructions for Use for more information on warnings, precautions and adverse events: ifu.sw-reducer.com
1: Abdallah, M. J Am Coll Cardiol 2017;69:2039-50.
2: Stone, G. 2-year results from the ABSORB IV randomized trial. TCT 2019.
3: Patel, M. N Engl J Med 2010; 362:886-895.
4: Samuels, B. J Am Coll Cardiol 2023; 82:1245-1263.
5: Verheye, S. Results from the REDUCER-I Study. ESC 2024.
6: Verheye S, Jolicoeur EM, Behan MW, et al. Efficacy of a device to narrow the coronary sinus in refractory angina. N Engl J Med 2015; 372: 519-527.
7: Foley et al. The Lancet. 2024 Apr 8; https://doi.org/10.1016/S0140-6736(24)00256-3.