USA / International

Shockwave EU Post-Market Study Shows Strong Safety and Procedural Success
Key Findings
Disrupt CAD II Study confirms promising CAD I results and continues to demonstrate promising safety outcomes in more centers and more patients:
- Low rate of in-hospital MACE (5.8%)
- No perforations, slow flow, no-reflow or major (Type D-F) dissections
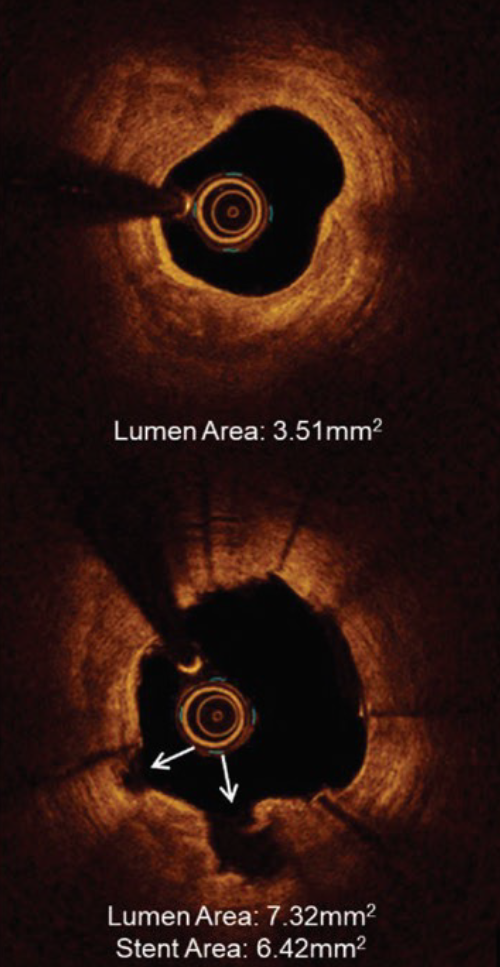
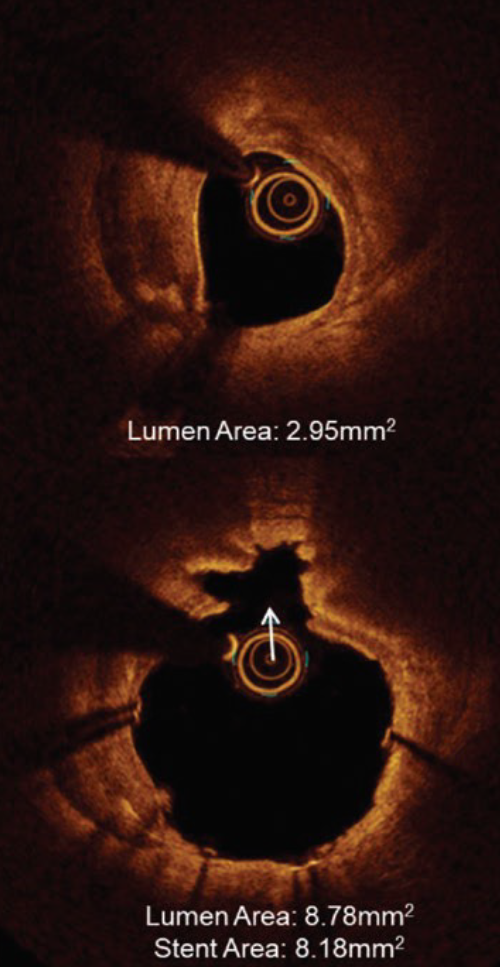
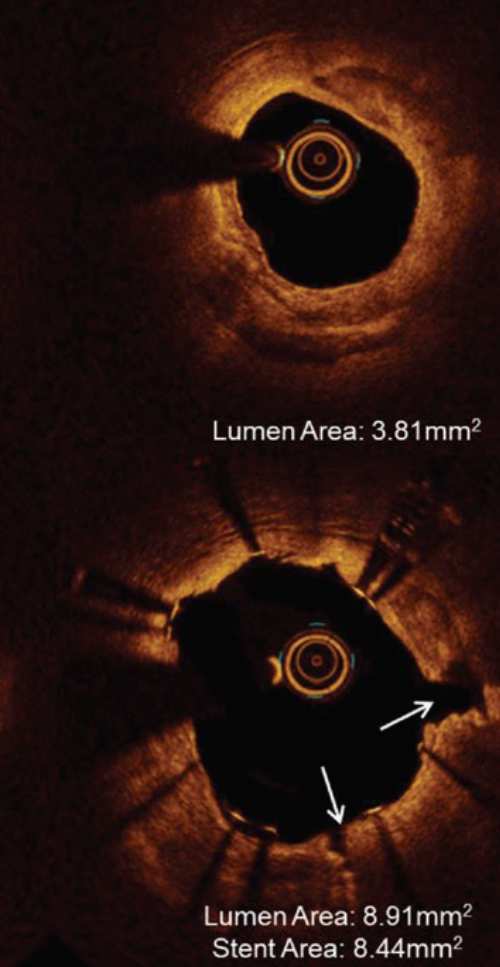
IVL optimizes clinical outcomes in significantly calcified lesions
- 100% successful delivery of IVL
- 100% successful delivery of stents with high acute gain (1.7mm2) and low residual stenosis (8%)