
Count On It:
New Data Confirms IVL’s Impact Below the Knee (BTK)
Acute and 30-Day Outcomes
ENDPOINTS
Primary safety endpoint: Major adverse limb events (MALE) or post-operative death (POD) at 30-days
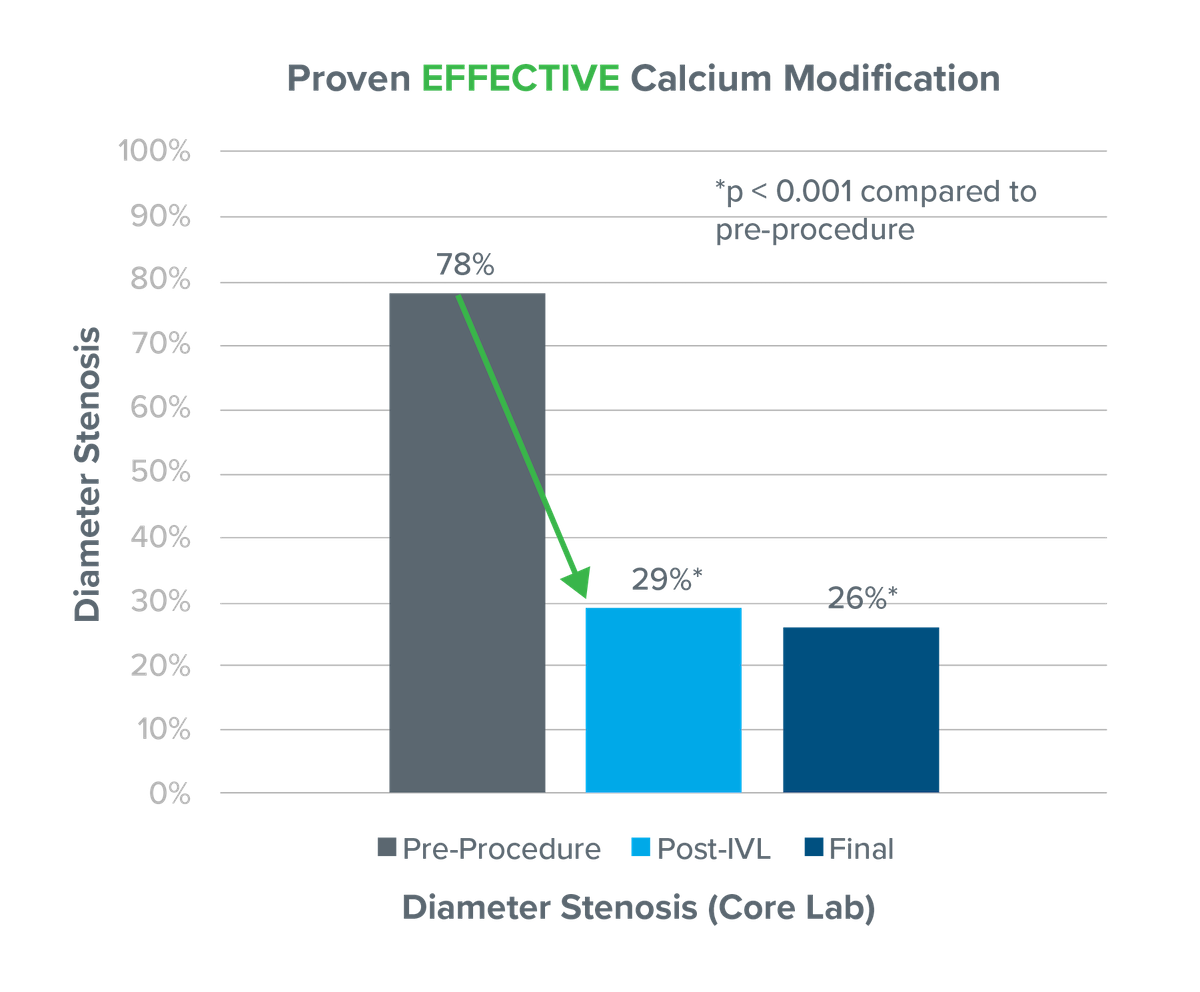
Primary effectiveness endpoint: Procedural success, defined as ≤ 50% residual stenosis for all treated target lesions without serious angiographic complications
Additional analysis to include lesions with ≤ 30% residual stenosis without angiographic complications
Independent Clinical Events Committee (CEC), Angiographic Core Laboratory, and Duplex Ultrasound Core Laboratory
Assess the continued safety and effectiveness of the Shockwave Medical peripheral IVL system for the treatment of calcified, stenotic lesions in BTK arteries
Prospective
Multi-center
Single-arm
Products included Shockwave M5+ and Shockwave S4
RC 3-5, RC 3 capped at 20% of enrolled patients
Moderate-severe calcification*
Up to two BTK lesions ≤ 200 mm in length
* Presence of fluoroscopic evidence of calcification by PARC definition: 1) on parallel sides of the vessel and 2) extending > 50% the length of the lesion if lesion is ≥ 50 mm in length; or extending for minimum of 20 mm if lesion is < 50 mm in length
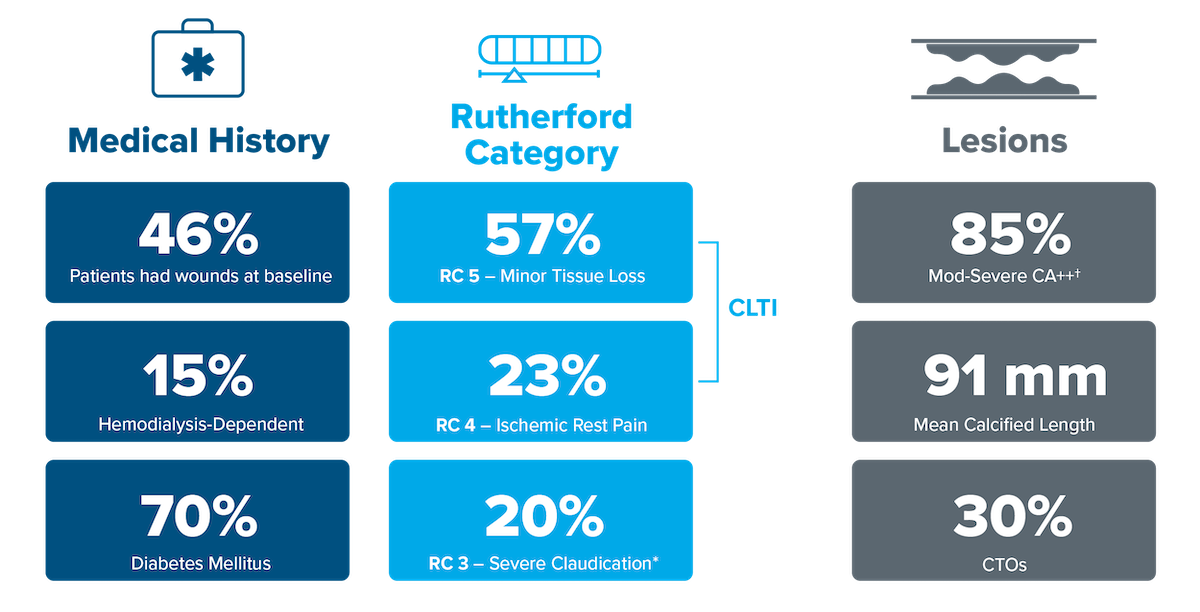
*Enrollment of RC3 was capped at 20% | †PARC Definition
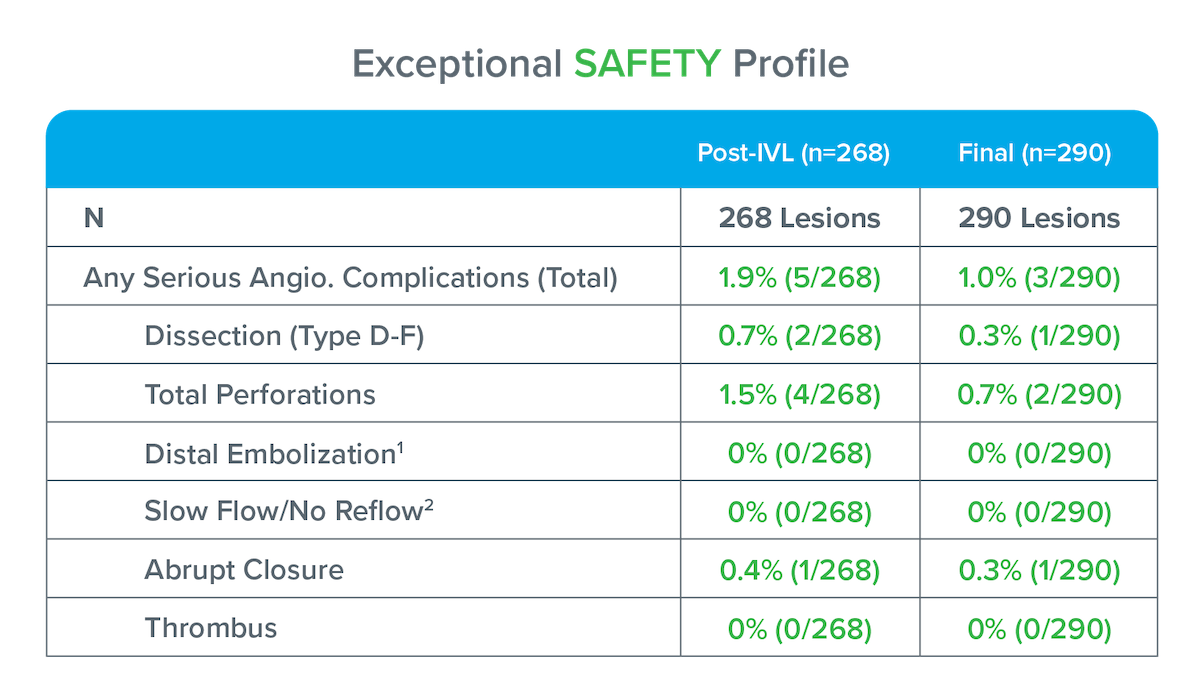 Three patients with serious angiographic complications at final:
Three patients with serious angiographic complications at final:
1: One patient had a distal thromboembolism post-IVL. Transluminal suction thrombectomy was performed and the event was successfully resolved although imaging was not provided to the core lab for assessment, therefore, data was not included in table.
2: No Reflow defined as reduced antegrade flow without evidence of residual stenosis or dissection at the treatment site.

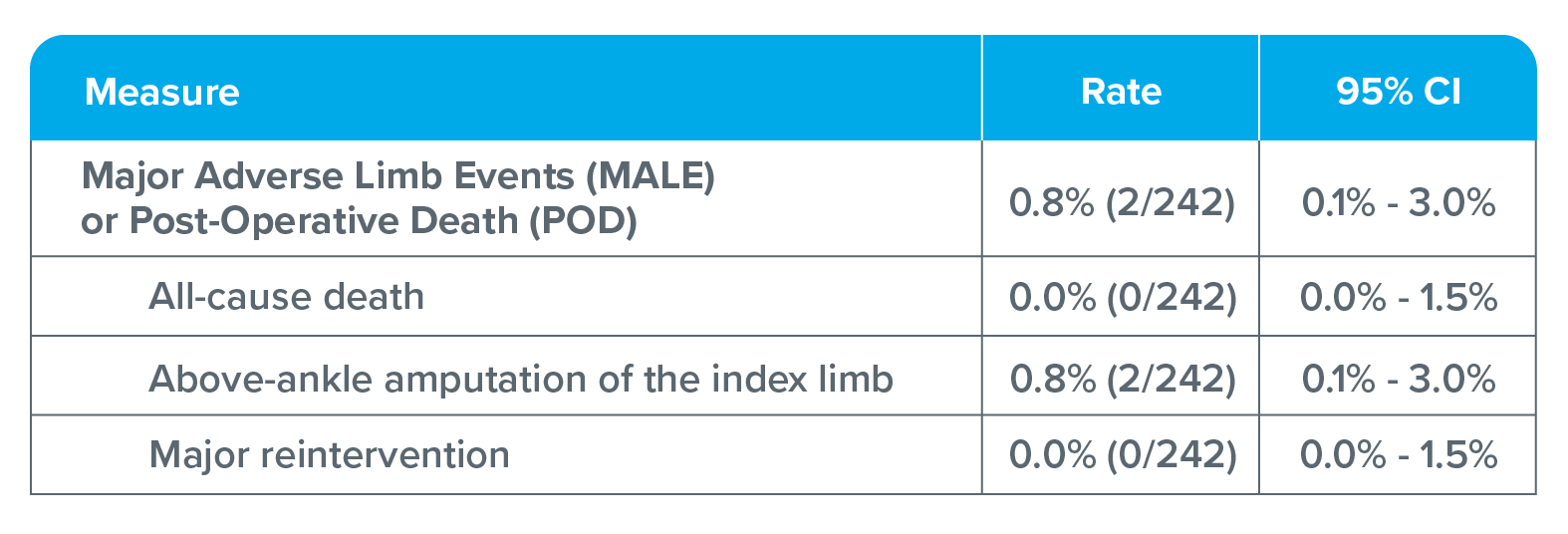 Two patients required above-ankle amputations of the index limb:
Two patients required above-ankle amputations of the index limb:
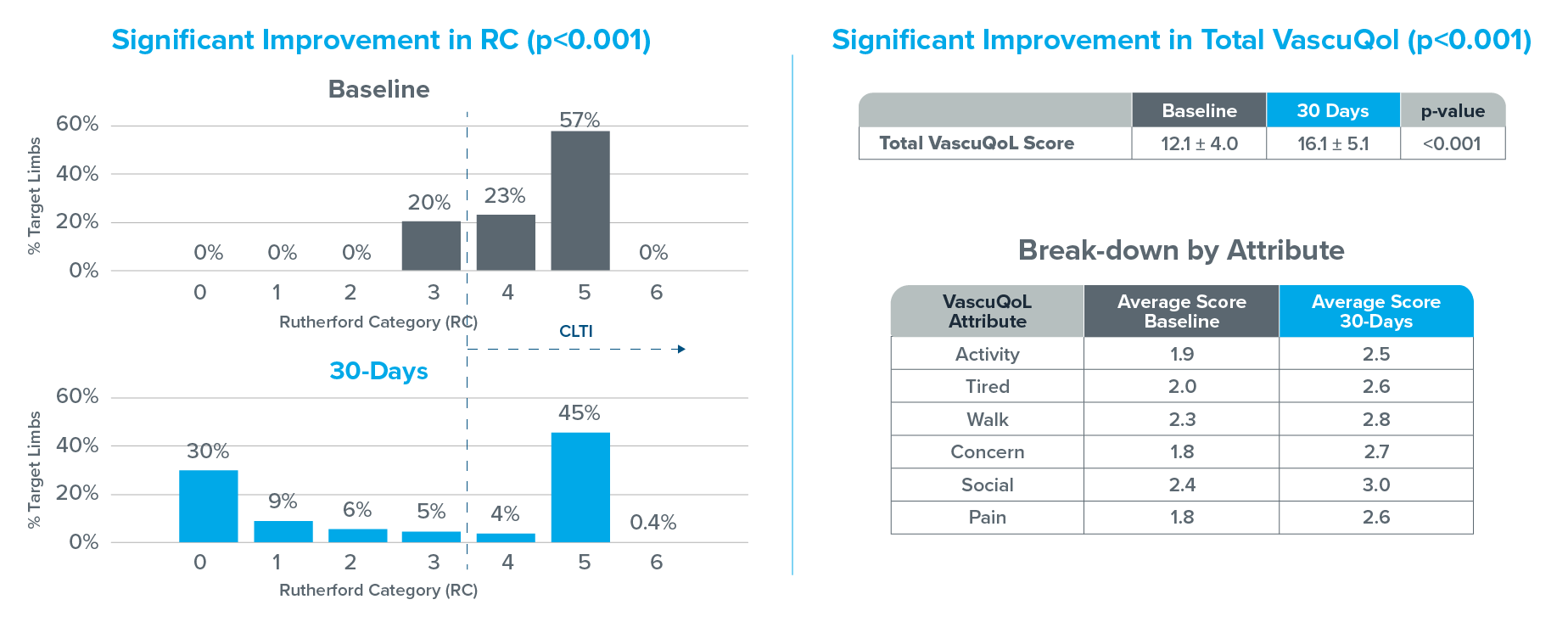
The Vascular Quality of Life Questionnaire is a survey used to determine health related quality of life (HRQoL) in patients with peripheral arterial disease (PAD). Each attribute is scored 1-4, a higher value indicates better health status. The sum of each individual attribute score is used to generate a total quality of life score.

Chandra V, VIVA Late Breaking Clinical Trial 2024
Looking for careers? Click here.